当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Intramolecular Oxa-Michael Reaction to α,β-Unsaturated Esters and Amides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-30 , DOI: 10.1021/jacs.3c03182 Guanglong Su 1 , Michele Formica 1 , Ken Yamazaki 1, 2 , Trevor A Hamlin 2 , Darren J Dixon 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-30 , DOI: 10.1021/jacs.3c03182 Guanglong Su 1 , Michele Formica 1 , Ken Yamazaki 1, 2 , Trevor A Hamlin 2 , Darren J Dixon 1
Affiliation
|
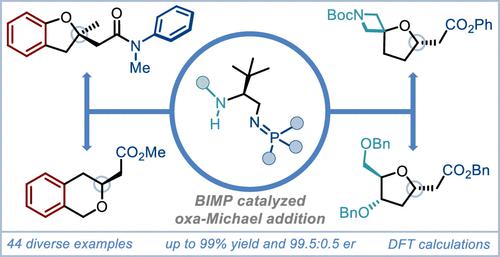
A bifunctional iminophosphorane (BIMP)-catalyzed, enantioselective intramolecular oxa-Michael reaction of alcohols to tethered, low electrophilicity Michael acceptors is described. Improved reactivity over previous reports (1 day vs 7 days), excellent yields (up to 99%), and enantiomeric ratios (up to 99.5:0.5 er) are demonstrated. The broad reaction scope, enabled by catalyst modularity and tunability, includes substituted tetrahydrofurans (THFs) and tetrahydropyrans (THPs), oxaspirocycles, sugar and natural product derivatives, dihydro-(iso)-benzofurans, and iso-chromans. A state-of-the-art computational study revealed that the enantioselectivity originates from the presence of several favorable intermolecular hydrogen bonds between the BIMP catalyst and the substrate that induce stabilizing electrostatic and orbital interactions. The newly developed catalytic enantioselective approach was carried out on multigram scale, and multiple Michael adducts were further derivatized to an array of useful building blocks, providing access to enantioenriched biologically active molecules and natural products.
中文翻译:

催化对映选择性分子内 Oxa-Michael 反应生成 α,β-不饱和酯和酰胺
描述了双功能亚氨基正膦 (BIMP) 催化的醇与束缚的低亲电性 Michael 受体的对映选择性分子内 oxa-Michael 反应。与之前的报告相比,反应性有所提高(1 天与 7 天相比)、优异的产率(高达 99%)和对映体比率(高达 99.5:0.5 er)。由于催化剂的模块化和可调性,反应范围广泛,包括取代的四氢呋喃 (THF) 和四氢吡喃 (THP)、氧杂螺环、糖和天然产物衍生物、二氢-(异)-苯并呋喃和异苯并二氢吡喃。最先进的计算研究表明,对映选择性源于 BIMP 催化剂和基材之间存在的几个有利的分子间氢键,从而诱导稳定的静电和轨道相互作用。
更新日期:2023-05-30
中文翻译:

催化对映选择性分子内 Oxa-Michael 反应生成 α,β-不饱和酯和酰胺
描述了双功能亚氨基正膦 (BIMP) 催化的醇与束缚的低亲电性 Michael 受体的对映选择性分子内 oxa-Michael 反应。与之前的报告相比,反应性有所提高(1 天与 7 天相比)、优异的产率(高达 99%)和对映体比率(高达 99.5:0.5 er)。由于催化剂的模块化和可调性,反应范围广泛,包括取代的四氢呋喃 (THF) 和四氢吡喃 (THP)、氧杂螺环、糖和天然产物衍生物、二氢-(异)-苯并呋喃和异苯并二氢吡喃。最先进的计算研究表明,对映选择性源于 BIMP 催化剂和基材之间存在的几个有利的分子间氢键,从而诱导稳定的静电和轨道相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号