当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of Ring-Opening of Tetrahydrofurfuryl Alcohol over WOx-Modified Pt Model Surfaces and Powder Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-30 , DOI: 10.1021/acscatal.3c01287 Zhexi Lin 1, 2 , Siddharth Deshpande 2, 3 , Steven R. Denny 1, 2 , William N. Porter 1 , Cong Wang 2 , Justin Marlowe 4 , Phillip Christopher 4 , Weiqing Zheng 2 , Stavros Caratzoulas 2 , Dionisios G. Vlachos 2, 3 , Jingguang G. Chen 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-30 , DOI: 10.1021/acscatal.3c01287 Zhexi Lin 1, 2 , Siddharth Deshpande 2, 3 , Steven R. Denny 1, 2 , William N. Porter 1 , Cong Wang 2 , Justin Marlowe 4 , Phillip Christopher 4 , Weiqing Zheng 2 , Stavros Caratzoulas 2 , Dionisios G. Vlachos 2, 3 , Jingguang G. Chen 1, 2
Affiliation
|
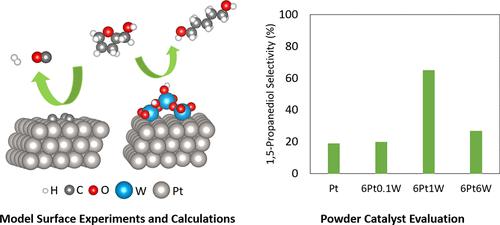
The upgrading of heterocyclic biomass-derived oxygenates such as tetrahydrofurfuryl alcohol (THFA) via ring-opening is a promising pathway to produce value-added diol molecules using renewable carbon sources. This study combines model surface experiments, first-principles calculations, and powder catalyst characterization and activity evaluation to unravel the nature of the Pt and WOx active sites and the reaction mechanism of the THFA ring-opening reaction on a WOx/Pt inverse oxide catalyst. Temperature-programmed desorption (TPD) and high-resolution electron energy loss spectroscopy (HREELS) measurements on model surfaces demonstrated that THFA ring opened on Pt(111) but underwent further decomposition due to its strong bonding with the surface. However, WOx deposited on Pt(111) altered the interaction strength between the ring-opened intermediate and the surface to a proper extent to facilitate the facile desorption of the desired 1,5-pentanediol (1,5-PeD) product. Density functional theory (DFT) calculations showed that WOx/Pt(111) could promote ring opening of THFA via an oxocarbenium ion-like transition state, which was stabilized by hydrogen bonding with the hydroxyl groups of WOx. The hydrogenation of the ring-opened 5-hydroxyvaleraldehyde intermediate to 1,5-PeD was then feasible via Brønsted acid sites present on WOx. Steady-state activity studies on the corresponding powder catalysts showed that the 1,5-PeD selectivity increased from 20% on Pt/SiO2 to 65% on WOx/Pt/SiO2 with 1 wt % WOx loading, consistent with model surface experiments and DFT calculations. This study demonstrates the feasibility of using model surface experiments and first-principles calculations to guide practical catalyst design, and provides a design strategy that can be applied to the selective ring-opening of relevant heterocyclic biomass-derived oxygenates.
中文翻译:

四氢糠醇在 WOx 修饰的 Pt 模型表面和粉末催化剂上开环的机理理解
通过开环对杂环生物质衍生的含氧化合物(例如四氢糠醇(THFA))进行升级是利用可再生碳源生产增值二醇分子的一条有前途的途径。本研究结合模型表面实验、第一性原理计算、粉末催化剂表征和活性评估,揭示了 Pt 和 WO x活性位点的性质以及 WO x /Pt 反氧化物上 THFA 开环反应的反应机制催化剂。模型表面的程序升温解吸 (TPD) 和高分辨率电子能量损失谱 (HREELS) 测量表明,THFA 环在 Pt(111) 上打开,但由于其与表面的牢固结合而进一步分解。然而,WOx沉积在 Pt(111) 上的纳米粒子在适当程度上改变了开环中间体与表面之间的相互作用强度,以促进所需 1,5-戊二醇 (1,5-PeD) 产物的轻松解吸。密度泛函理论(DFT)计算表明,WO x /Pt(111)可以通过类氧碳鎓离子过渡态促进THFA开环,该过渡态通过与WO x的羟基形成氢键而稳定。然后,通过 WO x上存在的布朗斯台德酸位点,可以将开环的 5-羟基戊醛中间体氢化为 1,5-PeD 。对相应粉末催化剂的稳态活性研究表明,1,5-PeD选择性从Pt/SiO 2上的20%提高到WO x上的65%/Pt/SiO 2具有 1 wt% WO x负载量,与模型表面实验和 DFT 计算一致。这项研究证明了使用模型表面实验和第一性原理计算来指导实际催化剂设计的可行性,并提供了一种可应用于相关杂环生物质衍生含氧化合物选择性开环的设计策略。
更新日期:2023-05-30
中文翻译:

四氢糠醇在 WOx 修饰的 Pt 模型表面和粉末催化剂上开环的机理理解
通过开环对杂环生物质衍生的含氧化合物(例如四氢糠醇(THFA))进行升级是利用可再生碳源生产增值二醇分子的一条有前途的途径。本研究结合模型表面实验、第一性原理计算、粉末催化剂表征和活性评估,揭示了 Pt 和 WO x活性位点的性质以及 WO x /Pt 反氧化物上 THFA 开环反应的反应机制催化剂。模型表面的程序升温解吸 (TPD) 和高分辨率电子能量损失谱 (HREELS) 测量表明,THFA 环在 Pt(111) 上打开,但由于其与表面的牢固结合而进一步分解。然而,WOx沉积在 Pt(111) 上的纳米粒子在适当程度上改变了开环中间体与表面之间的相互作用强度,以促进所需 1,5-戊二醇 (1,5-PeD) 产物的轻松解吸。密度泛函理论(DFT)计算表明,WO x /Pt(111)可以通过类氧碳鎓离子过渡态促进THFA开环,该过渡态通过与WO x的羟基形成氢键而稳定。然后,通过 WO x上存在的布朗斯台德酸位点,可以将开环的 5-羟基戊醛中间体氢化为 1,5-PeD 。对相应粉末催化剂的稳态活性研究表明,1,5-PeD选择性从Pt/SiO 2上的20%提高到WO x上的65%/Pt/SiO 2具有 1 wt% WO x负载量,与模型表面实验和 DFT 计算一致。这项研究证明了使用模型表面实验和第一性原理计算来指导实际催化剂设计的可行性,并提供了一种可应用于相关杂环生物质衍生含氧化合物选择性开环的设计策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号