当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Personalized Cancer Vaccine that Induces Synergistic Innate and Adaptive Immune Responses
Advanced Materials ( IF 29.4 ) Pub Date : 2023-05-30 , DOI: 10.1002/adma.202303080 Da-Sol Kuen 1 , Jihye Hong 2 , Suyoung Lee 1 , Choong-Hyun Koh 1 , Minkyeong Kwak 3 , Byung-Seok Kim , Mungyo Jung 4 , Yoon-Joo Kim 5 , Byung-Sik Cho 4 , Byung-Soo Kim 2, 3, 6 , Yeonseok Chung 1
Advanced Materials ( IF 29.4 ) Pub Date : 2023-05-30 , DOI: 10.1002/adma.202303080 Da-Sol Kuen 1 , Jihye Hong 2 , Suyoung Lee 1 , Choong-Hyun Koh 1 , Minkyeong Kwak 3 , Byung-Seok Kim , Mungyo Jung 4 , Yoon-Joo Kim 5 , Byung-Sik Cho 4 , Byung-Soo Kim 2, 3, 6 , Yeonseok Chung 1
Affiliation
|
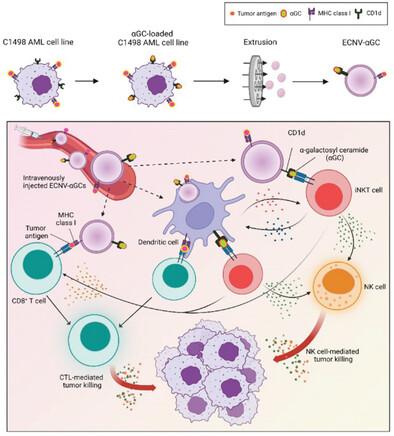
To demonstrate potent efficacy, a cancer vaccine needs to activate both innate and adaptive immune cells. Personalized cancer vaccine strategies often require the identification of patient-specific neoantigens; however, the clonal and mutational heterogeneity of cancer cells presents inherent challenges. Here, extracellular nanovesicles derived from alpha-galactosylceramide-conjugated autologous acute myeloid leukemia (AML) cells (ECNV-αGC) are presented as a personalized therapeutic vaccine that activates both innate and adaptive immune responses, bypassing the need to identify patient-specific neoantigens. ECNV-αGC vaccination directly engages with and activates both invariant natural killer T (iNKT) cells and leukemia-specific CD8+ T cells in mice with AML, thereby promoting long-term anti-leukemic immune memory. ECNV-αGC sufficiently serves as an antigen-presenting platform that can directly activate antigen-specific CD8+ T cells even in the absence of dendritic cells, thereby demonstrating a multifaceted cellular mechanism of immune activation. Moreover, ECNV-αGC vaccination results in a significantly lower AML burden and higher percentage of leukemia-free survivors among cytarabine-treated hosts with AML. Human AML-derived ECNV-αGCs activate iNKT cells in both healthy individuals and patients with AML regardless of responsiveness to conventional therapies. Together, autologous AML-derived ECNV-αGCs may be a promising personalized therapeutic vaccine that efficiently establishes AML-specific long-term immunity without requiring the identification of neoantigens.
中文翻译:

一种可诱导协同先天和适应性免疫反应的个性化癌症疫苗
为了证明有效的功效,癌症疫苗需要激活先天性和适应性免疫细胞。个性化癌症疫苗策略通常需要识别患者特异性新抗原;然而,癌细胞的克隆和突变异质性提出了固有的挑战。在这里,源自α-半乳糖神经酰胺缀合的自体急性髓性白血病(AML)细胞(ECNV- αGC)的细胞外纳米囊泡被作为一种个性化治疗疫苗,可激活先天性和适应性免疫反应,无需识别患者特异性新抗原。ECNV- α GC 疫苗接种直接参与并激活AML 小鼠中的不变自然杀伤 T (iNKT) 细胞和白血病特异性 CD8 + T 细胞,从而促进长期抗白血病免疫记忆。ECNV- αGC足以作为抗原呈递平台,即使在没有树突状细胞的情况下也可以直接激活抗原特异性CD8 + T细胞,从而展示了免疫激活的多方面细胞机制。此外,在接受阿糖胞苷治疗的 AML 宿主中, ECNV- α GC 疫苗接种可显着降低 AML 负担,并提高无白血病幸存者的比例。无论对传统疗法的反应如何,人类 AML 衍生的 ECNV- α GC 都会激活健康个体和 AML 患者的 iNKT 细胞。总之,自体 AML 衍生的 ECNV- α GC 可能是一种有前途的个性化治疗疫苗,可有效建立 AML 特异性长期免疫,而无需鉴定新抗原。
更新日期:2023-05-30
中文翻译:

一种可诱导协同先天和适应性免疫反应的个性化癌症疫苗
为了证明有效的功效,癌症疫苗需要激活先天性和适应性免疫细胞。个性化癌症疫苗策略通常需要识别患者特异性新抗原;然而,癌细胞的克隆和突变异质性提出了固有的挑战。在这里,源自α-半乳糖神经酰胺缀合的自体急性髓性白血病(AML)细胞(ECNV- αGC)的细胞外纳米囊泡被作为一种个性化治疗疫苗,可激活先天性和适应性免疫反应,无需识别患者特异性新抗原。ECNV- α GC 疫苗接种直接参与并激活AML 小鼠中的不变自然杀伤 T (iNKT) 细胞和白血病特异性 CD8 + T 细胞,从而促进长期抗白血病免疫记忆。ECNV- αGC足以作为抗原呈递平台,即使在没有树突状细胞的情况下也可以直接激活抗原特异性CD8 + T细胞,从而展示了免疫激活的多方面细胞机制。此外,在接受阿糖胞苷治疗的 AML 宿主中, ECNV- α GC 疫苗接种可显着降低 AML 负担,并提高无白血病幸存者的比例。无论对传统疗法的反应如何,人类 AML 衍生的 ECNV- α GC 都会激活健康个体和 AML 患者的 iNKT 细胞。总之,自体 AML 衍生的 ECNV- α GC 可能是一种有前途的个性化治疗疫苗,可有效建立 AML 特异性长期免疫,而无需鉴定新抗原。



























 京公网安备 11010802027423号
京公网安备 11010802027423号