当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO Oxidation on Ir1/TiO2: Resolving Ligand Dynamics and Elementary Reaction Steps
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-29 , DOI: 10.1021/acscatal.3c01433 Coogan B. Thompson 1 , Liping Liu 1 , Denis S. Leshchev 2 , Adam S. Hoffman 3 , Jiyun Hong 3 , Simon R. Bare 3 , Raymond R. Unocic 4 , Eli Stavitski 2 , Hongliang Xin 1 , Ayman M. Karim 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-29 , DOI: 10.1021/acscatal.3c01433 Coogan B. Thompson 1 , Liping Liu 1 , Denis S. Leshchev 2 , Adam S. Hoffman 3 , Jiyun Hong 3 , Simon R. Bare 3 , Raymond R. Unocic 4 , Eli Stavitski 2 , Hongliang Xin 1 , Ayman M. Karim 1
Affiliation
|
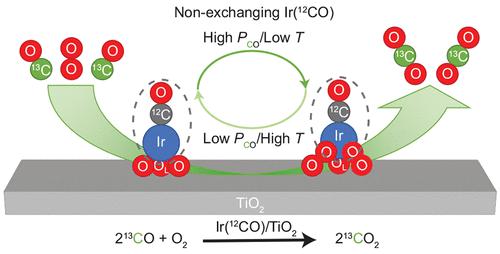
Identifying the rate-controlling steps and the evolution of the ligand environment throughout the catalytic cycle on supported single-atom catalysts is crucial to bridge the gap between heterogeneous and homogeneous catalysis. Here we identified the rate-controlling elementary steps for CO oxidation on TiO2-supported Ir single atoms and isolated the corresponding intermediate Ir complexes. Kinetic measurements, operando spectroscopy, and quantum-chemical calculations indicate that the reaction mechanism has two kinetically relevant steps, CO adsorption/oxidation and O2 dissociation. By varying the reaction conditions, three Ir1 complexes (states) along the reaction cycle were isolated and identified using in situ spectroscopy. Furthermore, we show that all the intermediate Ir1 states share a common CO ligand that does not turn over. This study provides atomic level details on the active, intermediate complexes and reaction cycle of supported single-metal-atom catalysts, thereby offering future possibilities to control the ligand environment and reactivity.
中文翻译:

Ir1/TiO2 上的 CO 氧化:解析配体动力学和基本反应步骤
确定负载型单原子催化剂整个催化循环中的速率控制步骤和配体环境的演变对于弥合多相和均相催化之间的差距至关重要。在这里,我们确定了 TiO 2负载的 Ir 单原子上 CO 氧化的速率控制基本步骤,并分离了相应的中间体 Ir 配合物。动力学测量、操作光谱和量子化学计算表明反应机理有两个动力学相关的步骤,CO吸附/氧化和O 2解离。通过改变反应条件,三个 Ir 1使用原位光谱法分离并鉴定了反应周期中的配合物(状态)。此外,我们表明所有中间 Ir 1态共享一个不翻转的共同 CO 配体。这项研究提供了负载型单金属原子催化剂的活性、中间配合物和反应循环的原子级详细信息,从而为未来控制配体环境和反应性提供了可能性。
更新日期:2023-05-29
中文翻译:

Ir1/TiO2 上的 CO 氧化:解析配体动力学和基本反应步骤
确定负载型单原子催化剂整个催化循环中的速率控制步骤和配体环境的演变对于弥合多相和均相催化之间的差距至关重要。在这里,我们确定了 TiO 2负载的 Ir 单原子上 CO 氧化的速率控制基本步骤,并分离了相应的中间体 Ir 配合物。动力学测量、操作光谱和量子化学计算表明反应机理有两个动力学相关的步骤,CO吸附/氧化和O 2解离。通过改变反应条件,三个 Ir 1使用原位光谱法分离并鉴定了反应周期中的配合物(状态)。此外,我们表明所有中间 Ir 1态共享一个不翻转的共同 CO 配体。这项研究提供了负载型单金属原子催化剂的活性、中间配合物和反应循环的原子级详细信息,从而为未来控制配体环境和反应性提供了可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号