当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modifying Copper Local Environment with Electrolyte Additives to Alter CO2 Electroreduction vs Hydrogen Evolution
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-26 , DOI: 10.1021/acscatal.3c00035 Samaneh Sharifi Golru 1, 2 , Andrew S. May 1 , Elizabeth J. Biddinger 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2023-05-26 , DOI: 10.1021/acscatal.3c00035 Samaneh Sharifi Golru 1, 2 , Andrew S. May 1 , Elizabeth J. Biddinger 1, 2
Affiliation
|
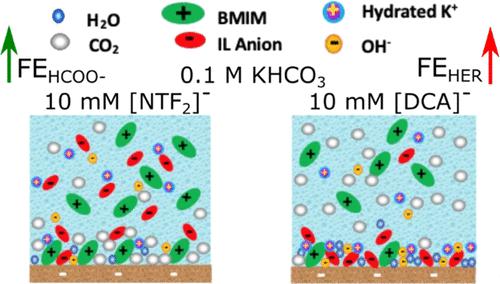
CO2 electroreduction (CO2ER) by using renewable energy resources is a promising method to mitigate the CO2 level in the atmosphere as well as produce valuable chemicals. Local environment at the electrode–electrolyte interface plays a key role in CO2ER activity and selectivity along with its competing hydrogen evolution reaction (HER). In addition to the catalyst and reactor design, electrolyte also has a significant impact on the interface. Herein, electrolyte additives were used to modify the local environment around the Cu catalyst during CO2ER. For this purpose, 10 mM ionic additives with bis(trifluoromethylsulfonyl)imide ([NTF2]−) and dicyanamide ([DCA]−) as anions and 1-butyl-3-methylimidazolium ([BMIM]+), potassium (K+), or sodium (Na+) as cations have been added to an aqueous potassium bicarbonate solution (0.1 M KHCO3). COMSOL Multiphysics was also used to calculate the local pH and CO2 concentration at the electrode–electrolyte interface in different electrolytes. Results showed that the local environment modifications by the electrolyte additives altered the activity and selectivity of Cu in CO2ER. It was found that the CO2ER activity at −0.92 V was enhanced when using anions with high CO2 affinity and high hydrophobicity, such as [NTF2]−. Among [NTF2]−-based additives, [BMIM][NTF2] had a higher faradaic efficiency (FE) for formate (38.7%) compared to K[NTF2] (23.2%) and Na[NTF2] (18.5%) at −0.92 V likely due to the presence of imidazolium cations that can further stabilize the intermediates on the surface and enhance CO2ER. Electrolytes containing [DCA]−-based additives with high hydrophilicity and low CO2 affinity had a very high HER selectivity (>90% FEH2) and low CO2ER selectivity regardless of the cation nature. This observation is attributed to the presence of hydrophilic [BMIM][DCA] in the vicinity of the catalyst, which impacts the microenvironment around the catalyst. We observed that [DCA]− anions have a high affinity to adsorb on Cu catalysts as soon as the catalyst is submerged in the electrolyte. Although FTIR showed that [DCA]− anions desorb from the surface at negative potentials, it is likely that [DCA]− anions still remain in the proximity of the electrode, next to the adsorbed cations, impacting the transport of H2O and CO2, and altering the product selectivity. COMSOL calculations showed that the local pH is directly proportional to the H2 evolution activity. Also, hydrophilic salts such as those with the [DCA]− anion had a more alkaline local pH, which led to a lower CO2 concentration in the vicinity of the catalyst.
中文翻译:

使用电解质添加剂改变铜的局部环境以改变 CO2 电还原与析氢
利用可再生能源进行CO 2电还原(CO2ER)是一种很有前景的方法,可以降低大气中的CO 2水平并生产有价值的化学品。电极-电解质界面的局部环境在 CO2ER 活性和选择性及其竞争性析氢反应 (HER) 中起着关键作用。除了催化剂和反应器设计之外,电解质对界面也有重大影响。在此,电解质添加剂用于改变 CO2ER 过程中 Cu 催化剂周围的局部环境。为此,使用双(三氟甲基磺酰基)亚胺 ([NTF 2 ] - ) 和二氰胺 ([DCA] - )的 10 mM 离子添加剂)作为阴离子和1-丁基-3-甲基咪唑鎓([BMIM] +)、钾(K +)或钠(Na +)作为阳离子已添加到碳酸氢钾水溶液(0.1 M KHCO 3)中。COMSOL Multiphysics 还用于计算不同电解质中电极-电解质界面的局部 pH 值和 CO 2浓度。结果表明,电解质添加剂对局部环境的改变改变了 CO2ER 中 Cu 的活性和选择性。研究发现,当使用具有高CO 2亲和力和高疏水性的阴离子(例如[NTF 2 ] - )时,-0.92 V下的CO2ER活性增强。其中[NTF 2] −基添加剂,与 K[NTF 2 ] (23.2%) 和 Na[NTF 2 ] (18.5%) 相比,[BMIM][NTF 2 ] 对甲酸盐具有更高的法拉第效率 (FE ) ( 38.7 % ) - 0.92 V 可能是由于咪唑鎓阳离子的存在可以进一步稳定表面上的中间体并增强 CO2ER。含有具有高亲水性和低CO 2亲和力的[DCA] -基添加剂的电解质具有非常高的HER选择性(>90%FE H2 )和低CO2ER选择性,无论阳离子性质如何。这一观察结果归因于催化剂附近存在亲水性[BMIM][DCA],这影响了催化剂周围的微环境。我们观察到 [DCA]−一旦催化剂浸没在电解液中,阴离子就具有高亲和力吸附在铜催化剂上。尽管 FTIR 显示 [DCA] -阴离子在负电位下从表面解吸,但 [DCA] -阴离子很可能仍然保留在电极附近,靠近吸附的阳离子,影响 H 2 O 和 CO的传输2、改变产物选择性。COMSOL 计算表明,局部 pH 值与 H 2析出活性成正比。此外,亲水盐(例如具有[DCA] -阴离子的盐)具有更碱性的局部pH,这导致催化剂附近的CO 2浓度较低。
更新日期:2023-05-26
中文翻译:

使用电解质添加剂改变铜的局部环境以改变 CO2 电还原与析氢
利用可再生能源进行CO 2电还原(CO2ER)是一种很有前景的方法,可以降低大气中的CO 2水平并生产有价值的化学品。电极-电解质界面的局部环境在 CO2ER 活性和选择性及其竞争性析氢反应 (HER) 中起着关键作用。除了催化剂和反应器设计之外,电解质对界面也有重大影响。在此,电解质添加剂用于改变 CO2ER 过程中 Cu 催化剂周围的局部环境。为此,使用双(三氟甲基磺酰基)亚胺 ([NTF 2 ] - ) 和二氰胺 ([DCA] - )的 10 mM 离子添加剂)作为阴离子和1-丁基-3-甲基咪唑鎓([BMIM] +)、钾(K +)或钠(Na +)作为阳离子已添加到碳酸氢钾水溶液(0.1 M KHCO 3)中。COMSOL Multiphysics 还用于计算不同电解质中电极-电解质界面的局部 pH 值和 CO 2浓度。结果表明,电解质添加剂对局部环境的改变改变了 CO2ER 中 Cu 的活性和选择性。研究发现,当使用具有高CO 2亲和力和高疏水性的阴离子(例如[NTF 2 ] - )时,-0.92 V下的CO2ER活性增强。其中[NTF 2] −基添加剂,与 K[NTF 2 ] (23.2%) 和 Na[NTF 2 ] (18.5%) 相比,[BMIM][NTF 2 ] 对甲酸盐具有更高的法拉第效率 (FE ) ( 38.7 % ) - 0.92 V 可能是由于咪唑鎓阳离子的存在可以进一步稳定表面上的中间体并增强 CO2ER。含有具有高亲水性和低CO 2亲和力的[DCA] -基添加剂的电解质具有非常高的HER选择性(>90%FE H2 )和低CO2ER选择性,无论阳离子性质如何。这一观察结果归因于催化剂附近存在亲水性[BMIM][DCA],这影响了催化剂周围的微环境。我们观察到 [DCA]−一旦催化剂浸没在电解液中,阴离子就具有高亲和力吸附在铜催化剂上。尽管 FTIR 显示 [DCA] -阴离子在负电位下从表面解吸,但 [DCA] -阴离子很可能仍然保留在电极附近,靠近吸附的阳离子,影响 H 2 O 和 CO的传输2、改变产物选择性。COMSOL 计算表明,局部 pH 值与 H 2析出活性成正比。此外,亲水盐(例如具有[DCA] -阴离子的盐)具有更碱性的局部pH,这导致催化剂附近的CO 2浓度较低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号