Food Hydrocolloids ( IF 10.7 ) Pub Date : 2023-05-26 , DOI: 10.1016/j.foodhyd.2023.108924 Ping Sun , Qin Zhang , Yu Zhao , Dongshun Zhao , Xiaohui Zhao , Lianzhou Jiang , Yan Zhang , Fei Wu , Xiaonan Sui
|
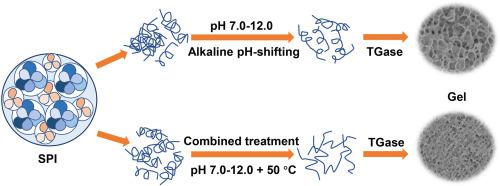
Improving the functional properties of soybean protein isolate (SPI) is essential for expanding its applications in food-related fields, given its poor solubility and gelation. This study investigated the effect of alkaline pH-shifting and mild heating on the structure of SPI, as well as the gel properties after TGase cross-linking. Solubility of SPI increased with increasing alkalinity from pH 7.0 to 12.0 at 25 °C, accompanied by decreased particle size and increased surface hydrophobicity. At pH 12.0 and 50 °C, SPI showed the highest solubility, smallest particle size, and best surface hydrophobicity. Intrinsic fluorescence spectra revealed that the protein structure unfolded after combined treatment, exposing more hydrophobic groups. TGase cross-linking modified SPI exhibited significantly increased gel hardness and water holding power. Therefore, alkaline pH transfer combined with mild heating treatment has great potential for generating functional modified SPI and provides a simple and easy method for preparing SPI with diverse gel and structural properties.
中文翻译:

通过碱性 pH 值变化、温和热处理和 TGase 交联改善大豆分离蛋白的凝胶特性
鉴于大豆分离蛋白 (SPI) 的溶解性和凝胶性较差,改善其功能特性对于扩大其在食品相关领域的应用至关重要。本研究调查了碱性 pH 值变化和温和加热对 SPI 结构的影响,以及 TGase 交联后的凝胶特性。在 25 °C 时,SPI 的溶解度随着碱度从 pH 7.0 增加到 12.0 而增加,同时粒径减小和表面疏水性增加。在 pH 12.0 和 50 °C 时,SPI 表现出最高的溶解度、最小的粒径和最佳的表面疏水性。内在荧光光谱显示,联合处理后蛋白质结构展开,暴露出更多的疏水基团。TGase 交联修饰的 SPI 表现出显着增加的凝胶硬度和保水力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号