当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aromaticity in Fully π-Conjugated Multicyclic Macrocycles
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-26 , DOI: 10.1021/jacs.3c03616 Longbin Ren 1 , Yi Han 1 , Xudong Hou 1 , Yong Ni 1 , Ya Zou 1 , Tianyu Jiao 1 , Jishan Wu 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-26 , DOI: 10.1021/jacs.3c03616 Longbin Ren 1 , Yi Han 1 , Xudong Hou 1 , Yong Ni 1 , Ya Zou 1 , Tianyu Jiao 1 , Jishan Wu 1
Affiliation
|
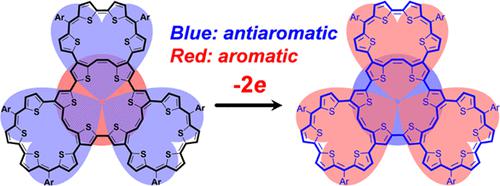
The research on aromaticity has mainly focused on monocyclic [n]annulene-like systems or polycyclic aromatic hydrocarbons. For fully π-conjugated multicyclic macrocycles (MMCs), the electronic coupling between the individual constitutional macrocycles would lead to unique electronic structures and aromaticity. The studies on MMCs, however, are quite limited, presumably due to the great challenges to design and synthesize a fully π-conjugated MMC molecule. Herein, we report the facile synthesis of two MMCs (2TMC and 3TMC) in which two and three thiophene-based macrocycles are fused together by employing both intramolecular and intermolecular Yamamoto coupling reactions of a properly designed precursor (7). The monocyclic macrocycle (1TMC) was also synthesized as a model compound. The geometry, aromaticity, and electronic properties of these macrocycles at different oxidation states were investigated by X-ray crystallographic analysis, NMR, and theoretical calculations, which disclosed how the constitutional macrocycles interplay with each other and lead to unique aromatic/antiaromatic character. This study provides new insights into the complex aromaticity in MMC systems.
中文翻译:

完全 π 共轭多环大环化合物的芳香性
芳香性的研究主要集中在单环[n]轮烯类系统或多环芳烃上。对于完全 π 共轭的多环大环化合物 (MMC),各个组成大环化合物之间的电子耦合将导致独特的电子结构和芳香性。然而,对 MMC 的研究非常有限,这可能是由于设计和合成完全 π 共轭的 MMC 分子面临巨大挑战。在此,我们报告了两种 MMC( 2TMC和3TMC)的简便合成,其中两个和三个基于噻吩的大环化合物通过使用适当设计的前体 ( 7 ) 的分子内和分子间 Yamamoto 偶联反应融合在一起。单环大环 ( 1TMC) 也被合成为模型化合物。通过 X 射线晶体学分析、核磁共振和理论计算研究了这些大环化合物在不同氧化态下的几何结构、芳香性和电子性质,揭示了构成大环化合物如何相互作用并导致独特的芳香/反芳香特性。这项研究为 MMC 系统中的复杂芳香性提供了新的见解。
更新日期:2023-05-26
中文翻译:

完全 π 共轭多环大环化合物的芳香性
芳香性的研究主要集中在单环[n]轮烯类系统或多环芳烃上。对于完全 π 共轭的多环大环化合物 (MMC),各个组成大环化合物之间的电子耦合将导致独特的电子结构和芳香性。然而,对 MMC 的研究非常有限,这可能是由于设计和合成完全 π 共轭的 MMC 分子面临巨大挑战。在此,我们报告了两种 MMC( 2TMC和3TMC)的简便合成,其中两个和三个基于噻吩的大环化合物通过使用适当设计的前体 ( 7 ) 的分子内和分子间 Yamamoto 偶联反应融合在一起。单环大环 ( 1TMC) 也被合成为模型化合物。通过 X 射线晶体学分析、核磁共振和理论计算研究了这些大环化合物在不同氧化态下的几何结构、芳香性和电子性质,揭示了构成大环化合物如何相互作用并导致独特的芳香/反芳香特性。这项研究为 MMC 系统中的复杂芳香性提供了新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号