当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cinchona-Alkaloid-Derived NN Ligands and Achiral Phosphines for Iridium-Catalyzed Asymmetric Hydrogenation of Heteroaromatic and α-Chloroheteroaryl Ketones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.joc.3c00786 Jie Tian 1 , Xin Meng 1 , Hao Sun 1 , Qian Chen 1 , Qian Xu 1 , Pinli Dai 1 , Linlin Li 1 , Lin Zhang 1 , Chun Li 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.joc.3c00786 Jie Tian 1 , Xin Meng 1 , Hao Sun 1 , Qian Chen 1 , Qian Xu 1 , Pinli Dai 1 , Linlin Li 1 , Lin Zhang 1 , Chun Li 1
Affiliation
|
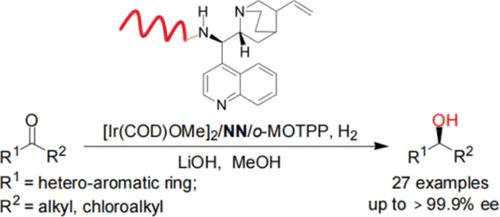
A concise synthesis of cinchona-alkaloid-derived NN ligands bearing alkyl substituents on chiral nitrogen atoms was described. Iridium catalysts containing new chiral NN ligands and achiral phosphines were effective for the asymmetric hydrogenation of heteroaromatic ketones, which afforded corresponding alcohols in up to 99.9% ee. The same protocol was applicable to the asymmetric hydrogenation of α-chloroheteroaryl ketones. Most importantly, the gram-scale asymmetric hydrogenation of 2-acetylthiophene and 2-acetylfuran proceeded smoothly even under 1 MPa of H2.
中文翻译:

金鸡纳生物碱衍生的 NN 配体和非手性膦用于铱催化杂芳酮和 α-氯杂芳基酮的不对称氢化
描述了手性氮原子上带有烷基取代基的金鸡纳生物碱衍生的 NN 配体的简明合成。含有新型手性 NN 配体和非手性膦的铱催化剂可有效地进行杂芳酮的不对称氢化,得到相应的醇,其 ee 高达 99.9%。相同的方案适用于α-氯杂芳基酮的不对称氢化。最重要的是,即使在1 MPa的H 2下,2-乙酰噻吩和2-乙酰呋喃的克级不对称氢化反应也能顺利进行。
更新日期:2023-05-25
中文翻译:

金鸡纳生物碱衍生的 NN 配体和非手性膦用于铱催化杂芳酮和 α-氯杂芳基酮的不对称氢化
描述了手性氮原子上带有烷基取代基的金鸡纳生物碱衍生的 NN 配体的简明合成。含有新型手性 NN 配体和非手性膦的铱催化剂可有效地进行杂芳酮的不对称氢化,得到相应的醇,其 ee 高达 99.9%。相同的方案适用于α-氯杂芳基酮的不对称氢化。最重要的是,即使在1 MPa的H 2下,2-乙酰噻吩和2-乙酰呋喃的克级不对称氢化反应也能顺利进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号