当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cation Co-Intercalation with Anions: The Origin of Low Capacities of Graphite Cathodes in Multivalent Electrolytes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c01555 Yuanyuan Yang 1, 2 , Jinzhi Wang 1 , Xiaofan Du 1, 3 , Hongzhu Jiang 1, 2 , Aobing Du 1 , Xuesong Ge 1 , Na Li 1 , Hao Wang 4 , Yuchen Zhang 1, 2 , Zheng Chen 1, 3 , Jingwen Zhao 1, 3 , Guanglei Cui 1, 5
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c01555 Yuanyuan Yang 1, 2 , Jinzhi Wang 1 , Xiaofan Du 1, 3 , Hongzhu Jiang 1, 2 , Aobing Du 1 , Xuesong Ge 1 , Na Li 1 , Hao Wang 4 , Yuchen Zhang 1, 2 , Zheng Chen 1, 3 , Jingwen Zhao 1, 3 , Guanglei Cui 1, 5
Affiliation
|
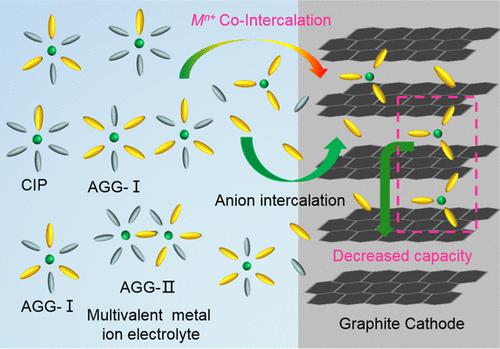
Dual-ion batteries involving anion intercalation into graphite cathodes represent promising battery technologies for low-cost and high-power energy storage. However, the fundamental origins regarding much lower capacities of graphite cathodes in earth abundant and inexpensive multivalent electrolytes than in Li-ion electrolytes remain elusive. Herein, we reveal that the limited anion-storage capacity of a graphite cathode in multivalent electrolytes is rooted in the abnormal multivalent-cation co-intercalation with anions in the form of large-sized anionic complexes. This cation co-intercalation behavior persists throughout the stage evolution of graphite intercalation compounds and leads to a significant decrease of sites practically viable for capacity contribution inside graphite galleries. Further systematic studies illustrate that the phenomenon of cation co-intercalation into graphite is closely related to the high energy penalty of interfacial anion desolvation due to the strong cation–anion association prevalent in multivalent electrolytes. Leveraging this understanding, we verify that promoting ionic dissociation in multivalent electrolytes by employing high-permittivity and oxidation-tolerant co-solvents is effective in suppressing multivalent-cation co-intercalation and thus achieving increased capacity of graphite cathodes. For instance, introducing adiponitrile as a co-solvent to a Mg2+-based carbonate electrolyte leads to 83% less Mg2+ co-intercalation and a ∼29.5% increase in delivered capacity of the graphite cathode.
中文翻译:

阳离子与阴离子的共嵌入:多价电解质中石墨阴极低容量的根源
将阴离子嵌入石墨阴极的双离子电池代表了用于低成本和高功率储能的有前途的电池技术。然而,与锂离子电解质相比,地球丰富且廉价的多价电解质中石墨阴极的容量低得多的基本起源仍然难以捉摸。在此,我们揭示了石墨正极在多价电解质中有限的阴离子存储能力,其根源在于多价阳离子与大尺寸阴离子络合物形式的阴离子的异常共嵌入。这种阳离子共嵌入行为在石墨层间化合物的整个阶段演化过程中持续存在,并导致石墨通道内实际可行的容量贡献显着减少。进一步的系统研究表明,由于多价电解质中普遍存在强阳离子-阴离子缔合,阳离子共嵌入石墨的现象与界面阴离子去溶剂化的高能量损失密切相关。利用这种理解,我们证实通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子解离可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg 我们证实,通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子离解可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg 我们证实,通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子离解可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg基于2+的碳酸盐电解质可使 Mg 2+共嵌入减少 83% ,并使石墨阴极的输送容量增加约 29.5%。
更新日期:2023-05-25
中文翻译:

阳离子与阴离子的共嵌入:多价电解质中石墨阴极低容量的根源
将阴离子嵌入石墨阴极的双离子电池代表了用于低成本和高功率储能的有前途的电池技术。然而,与锂离子电解质相比,地球丰富且廉价的多价电解质中石墨阴极的容量低得多的基本起源仍然难以捉摸。在此,我们揭示了石墨正极在多价电解质中有限的阴离子存储能力,其根源在于多价阳离子与大尺寸阴离子络合物形式的阴离子的异常共嵌入。这种阳离子共嵌入行为在石墨层间化合物的整个阶段演化过程中持续存在,并导致石墨通道内实际可行的容量贡献显着减少。进一步的系统研究表明,由于多价电解质中普遍存在强阳离子-阴离子缔合,阳离子共嵌入石墨的现象与界面阴离子去溶剂化的高能量损失密切相关。利用这种理解,我们证实通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子解离可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg 我们证实,通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子离解可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg 我们证实,通过使用高介电常数和抗氧化助溶剂促进多价电解质中的离子离解可有效抑制多价阳离子共嵌入,从而实现石墨阴极容量的增加。例如,将己二腈作为共溶剂引入 Mg基于2+的碳酸盐电解质可使 Mg 2+共嵌入减少 83% ,并使石墨阴极的输送容量增加约 29.5%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号