Water Research ( IF 12.8 ) Pub Date : 2023-05-25 , DOI: 10.1016/j.watres.2023.120131 Tianqi Zhang 1 , Urs von Gunten 2
|
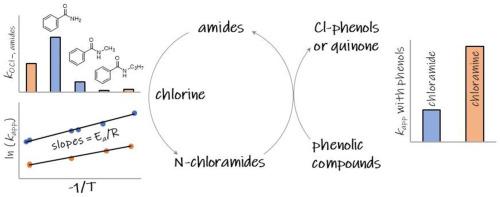
Amides are common constituents in natural organic matter and synthetic chemicals. In this study, we investigated kinetics and mechanisms of the reactions of chlorine with seven amides, including acetamide, N-methylformamide, N-methylacetamide, benzamide, N-methylbenzamide, N-propylbenzamide, and N-(benzoylglycyl)glycine amide. Apparent second-order rate constants for the reactions of the amides with chlorine at pH 8 are in the range of 5.8 × 10−3 – 1.8 M−1s−1 and activation energies in the range of 62-88 kJ/mol. The second-order rate constants for the reactions of chlorine with different amides decrease with increasing electron donor character of the substituents on the amide-N and N-carbonyl-C in the amide structures. Hypochlorite (‒OCl) dominates the reactions of chlorine with amides yielding N-chloramides with species-specific second-order rate constants in the range of 7.3 × 10−3 – 2.3 M−1s−1. Kinetic model simulations suggest that N-chlorinated primary amides further react with HOCl with second-order rate constants in the order of 10 M−1s−1. The chlorination products of amides, N-chloramides are reactive towards phenolic compounds, forming chlorinated phenols via electrophilic aromatic substitution (phenol and resorcinol) and quinone via electron transfer (hydroquinone). Meanwhile, N-chloramides were recycled to the parent amides. At neutral pH, apparent second-order rate constants for the reactions between phenols and N-chloramides are in the order of 10−4-0.1 M−1s−1, comparable to those with chloramine. The findings of this study improve the understanding of the fate of amides and chlorine during chlorination processes.
中文翻译:

酰胺的氯化:N-氯酰胺形成的动力学和机制及其与酚类化合物的反应
酰胺是天然有机物和合成化学品中的常见成分。在这项研究中,我们研究了氯与七种酰胺的反应动力学和机理,包括乙酰胺、N-甲基甲酰胺、N-甲基乙酰胺、苯甲酰胺、N-甲基苯甲酰胺、N-丙基苯甲酰胺和N- (苯甲酰甘氨酰)甘氨酰胺。在pH 8 下,酰胺与氯的反应的表观二阶速率常数在5.8 × 10 -3 – 1.8 M -1 s -1范围内,活化能在62-88 kJ/mol 范围内。氯与不同酰胺反应的二级速率常数随着酰胺结构中酰胺-N和N-羰基-C上取代基的电子给体特征的增加而降低。次氯酸盐 ( -OCl ) 在氯与酰胺的反应中占主导地位,生成N -氯酰胺,其物种特异性二阶速率常数在 7.3 × 10 -3 – 2.3 M -1 s -1范围内。动力学模型模拟表明N-氯化伯酰胺进一步与HOCl反应,二级速率常数约为10 M -1 s -1。酰胺、N-氯酰胺的氯化产物对酚类化合物具有反应性,通过亲电芳香取代(苯酚和间苯二酚)形成氯化酚,通过电子转移形成醌(氢醌)。同时,N-氯酰胺被回收为母体酰胺。在中性pH下,苯酚和N-氯酰胺之间的反应的表观二阶速率常数约为10 -4 -0.1 M -1 s -1,与氯胺的反应相当。这项研究的结果提高了人们对氯化过程中酰胺和氯的命运的了解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号