Journal of Hepatology ( IF 25.7 ) Pub Date : 2023-05-22 , DOI: 10.1016/j.jhep.2023.04.037 Elena Kotsiliti 1 , Valentina Leone 2 , Svenja Schuehle 3 , Olivier Govaere 4 , Hai Li 5 , Monika J Wolf 6 , Helena Horvatic 7 , Sandra Bierwirth 8 , Jana Hundertmark 9 , Donato Inverso 10 , Laimdota Zizmare 11 , Avital Sarusi-Portuguez 12 , Revant Gupta 13 , Tracy O'Connor 14 , Anastasios D Giannou 15 , Ahmad Mustafa Shiri 16 , Yehuda Schlesinger 12 , Maria Garcia Beccaria 1 , Charlotte Rennert 17 , Dominik Pfister 1 , Rupert Öllinger 18 , Iana Gadjalova 19 , Pierluigi Ramadori 1 , Mohammad Rahbari 1 , Nuh Rahbari 20 , Marc E Healy 21 , Mirian Fernández-Vaquero 1 , Neda Yahoo 1 , Jakob Janzen 1 , Indrabahadur Singh 22 , Chaofan Fan 1 , Xinyuan Liu 23 , Monika Rau 24 , Martin Feuchtenberger 25 , Eva Schwaneck 26 , Sebastian J Wallace 27 , Simon Cockell 28 , John Wilson-Kanamori 27 , Prakash Ramachandran 27 , Celia Kho 7 , Timothy J Kendall 29 , Anne-Laure Leblond 6 , Selina J Keppler 19 , Piotr Bielecki 30 , Katja Steiger 31 , Maike Hofmann 32 , Karsten Rippe 33 , Horst Zitzelsberger 34 , Achim Weber 6 , Nisar Malek 35 , Tom Luedde 36 , Mihael Vucur 36 , Hellmut G Augustin 10 , Richard Flavell 30 , Oren Parnas 37 , Roland Rad 38 , Olivier Pabst 39 , Neil C Henderson 29 , Samuel Huber 16 , Andrew Macpherson 5 , Percy Knolle 40 , Manfred Claassen 41 , Andreas Geier 24 , Christoph Trautwein 11 , Kristian Unger 34 , Eran Elinav 42 , Ari Waisman 23 , Zeinab Abdullah 7 , Dirk Haller 8 , Frank Tacke 9 , Quentin M Anstee 43 , Mathias Heikenwalder 44
|
Background & Aims
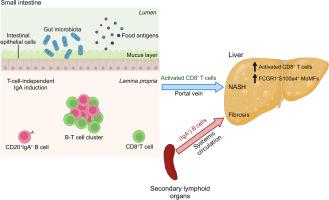
The progression of non-alcoholic steatohepatitis (NASH) to fibrosis and hepatocellular carcinoma (HCC) is aggravated by auto-aggressive T cells. The gut-liver axis contributes to NASH, but the mechanisms involved and the consequences for NASH-induced fibrosis and liver cancer remain unknown. We investigated the role of gastrointestinal B cells in the development of NASH, fibrosis and NASH-induced HCC.
Methods
C57BL/6J wild-type (WT), B cell-deficient and different immunoglobulin-deficient or transgenic mice were fed distinct NASH-inducing diets or standard chow for 6 or 12 months, whereafter NASH, fibrosis, and NASH-induced HCC were assessed and analysed. Specific pathogen-free/germ-free WT and μMT mice (containing B cells only in the gastrointestinal tract) were fed a choline-deficient high-fat diet, and treated with an anti-CD20 antibody, whereafter NASH and fibrosis were assessed. Tissue biopsy samples from patients with simple steatosis, NASH and cirrhosis were analysed to correlate the secretion of immunoglobulins to clinicopathological features. Flow cytometry, immunohistochemistry and single-cell RNA-sequencing analysis were performed in liver and gastrointestinal tissue to characterise immune cells in mice and humans.
Results
Activated intestinal B cells were increased in mouse and human NASH samples and licensed metabolic T-cell activation to induce NASH independently of antigen specificity and gut microbiota. Genetic or therapeutic depletion of systemic or gastrointestinal B cells prevented or reverted NASH and liver fibrosis. IgA secretion was necessary for fibrosis induction by activating CD11b+CCR2+F4/80+CD11c-FCGR1+ hepatic myeloid cells through an IgA-FcR signalling axis. Similarly, patients with NASH had increased numbers of activated intestinal B cells; additionally, we observed a positive correlation between IgA levels and activated FcRg+ hepatic myeloid cells, as well the extent of liver fibrosis.
Conclusions
Intestinal B cells and the IgA-FcR signalling axis represent potential therapeutic targets for the treatment of NASH.
Impact and Implications
There is currently no effective treatment for non-alcoholic steatohepatitis (NASH), which is associated with a substantial healthcare burden and is a growing risk factor for hepatocellular carcinoma (HCC). We have previously shown that NASH is an auto-aggressive condition aggravated, amongst others, by T cells. Therefore, we hypothesized that B cells might have a role in disease induction and progression. Our present work highlights that B cells have a dual role in NASH pathogenesis, being implicated in the activation of auto-aggressive T cells and the development of fibrosis via activation of monocyte-derived macrophages by secreted immunoglobulins (e.g., IgA). Furthermore, we show that the absence of B cells prevented HCC development. B cell-intrinsic signalling pathways, secreted immunoglobulins, and interactions of B cells with other immune cells are potential targets for combinatorial NASH therapies against inflammation and fibrosis.
中文翻译:

肠道 B 细胞独立于 NASH 微生物群/抗原许可代谢 T 细胞激活,并通过 IgA-FcR 信号传导促进纤维化
背景与目标
自身攻击性 T 细胞会加剧非酒精性脂肪性肝炎 (NASH) 向纤维化和肝细胞癌 (HCC) 的进展。肠-肝轴导致 NASH,但涉及的机制以及 NASH 诱导的纤维化和肝癌的后果仍然未知。我们研究了胃肠道 B 细胞在 NASH、纤维化和 NASH 诱导的 HCC 发展中的作用。
方法
C57BL/6J 野生型 (WT)、B 细胞缺陷和不同的免疫球蛋白缺陷或转基因小鼠被喂食不同的 NASH 诱导饮食或标准食物 6 或 12 个月,然后评估 NASH、纤维化和 NASH 诱导的 HCC并进行了分析。特定的无病原体/无菌 WT 和 μMT 小鼠(仅在胃肠道中含有 B 细胞)被喂食缺乏胆碱的高脂肪饮食,并用抗 CD20 抗体治疗,然后评估 NASH 和纤维化。对单纯性脂肪变性、NASH 和肝硬化患者的组织活检样本进行分析,将免疫球蛋白的分泌与临床病理特征相关联。在肝脏和胃肠组织中进行流式细胞术、免疫组织化学和单细胞 RNA 测序分析,以表征小鼠和人类的免疫细胞。
结果
小鼠和人类 NASH 样本中激活的肠道 B 细胞有所增加,并且获得许可的代谢 T 细胞激活可独立于抗原特异性和肠道微生物群诱导 NASH。通过基因或治疗方法消除全身或胃肠道 B 细胞可预防或逆转 NASH 和肝纤维化。IgA 分泌是通过 IgA-FcR 信号轴激活 CD11b+CCR2+F4/80+CD11c-FCGR1+ 肝骨髓细胞诱导纤维化所必需的。同样,NASH 患者的活化肠道 B 细胞数量也有所增加。此外,我们观察到 IgA 水平与活化的 FcRg+ 肝髓细胞以及肝纤维化程度之间呈正相关。
结论
肠道 B 细胞和 IgA-FcR 信号轴代表了 NASH 治疗的潜在治疗靶点。
影响和启示
目前,非酒精性脂肪性肝炎 (NASH) 尚无有效治疗方法,该病会造成巨大的医疗负担,并且是肝细胞癌 (HCC) 日益增长的危险因素。我们之前已经证明 NASH 是一种由 T 细胞等因素加剧的自身攻击性疾病。因此,我们假设 B 细胞可能在疾病的诱导和进展中发挥作用。我们目前的工作强调,B 细胞在 NASH 发病机制中具有双重作用,涉及自身攻击性 T 细胞的激活以及通过分泌的免疫球蛋白(例如 IgA)激活单核细胞衍生的巨噬细胞而导致纤维化的发展。此外,我们发现 B 细胞的缺失可以阻止 HCC 的发展。B 细胞内在信号通路、分泌的免疫球蛋白以及 B 细胞与其他免疫细胞的相互作用是针对炎症和纤维化的组合 NASH 疗法的潜在目标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号