Journal of Hepatology ( IF 25.7 ) Pub Date : 2023-05-22 , DOI: 10.1016/j.jhep.2023.04.031 Stephen A Harrison 1 , Vlad Ratziu 2 , Jeremy Magnanensi 3 , Yacine Hajji 3 , Sylvie Deledicque 3 , Zouher Majd 3 , Christian Rosenquist 3 , Dean W Hum 3 , Bart Staels 4 , Quentin M Anstee 5 , Arun J Sanyal 6
|
Background & Aims
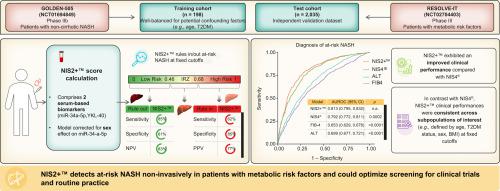
NIS4® is a blood-based non-invasive test designed to effectively rule in/rule out at-risk non-alcoholic steatohepatitis (NASH), defined as non-alcoholic fatty liver disease activity score ≥4 and significant fibrosis (stage ≥2), among patients with metabolic risk factors. Robustness of non-invasive test scores across characteristics of interest including age, type 2 diabetes mellitus, and sex, and optimised analytical aspects are critical for large-scale implementation in clinical practice. We developed and validated NIS2+™, an optimisation of NIS4®, specifically designed to improve score robustness.
Methods
A well-balanced training cohort (n = 198) included patients from the GOLDEN-505 trial. The validation (n = 684) and test (n = 2,035) cohorts included patients from the RESOLVE-IT trial. Well-matched subgroups were created to avoid potential confounding effects during modelling and analysis of score robustness. Models were trained using logistic regressions for at-risk NASH detection and compared using Bayesian information criteria. Performance of NIS2+™ was compared with that of NIS4®, Fibrosis-4, and alanine aminotransferase using area under the receiver operating characteristic curve, and robustness was analysed through score distribution.
Results
Using the training cohort to compare all combinations of NIS4® biomarkers, NIS2 (miR-34a-5p, YKL-40) was identified as the best combination of parameters. To correct for the sex effect on miR-34a-5p (validation cohort), sex and sex ∗ miR-34a-5p parameters were added, creating NIS2+™. In the test cohort, NIS2+™ exhibited a statistically higher area under the receiver operating characteristic curve (0.813) vs. NIS4® (0.792; p = 0.0002), Fibrosis-4 (0.653; p <0.0001), and alanine aminotransferase (0.699; p <0.0001). NIS2+™ scores were not affected by age, sex, BMI, or type 2 diabetes mellitus status, providing robust clinical performances irrespective of patient characteristics.
Conclusion
NIS2+™ constitutes a robust optimisation of NIS4® technology for the detection of at-risk NASH.
Impact and implications
The development of non-invasive tests for accurate, large-scale detection of patients with at-risk non-alcoholic steatohepatitis (NASH; defined as NASH with non-alcoholic fatty liver disease activity score ≥4 and fibrosis stage ≥2) – who are at higher risk for disease progression and for developing liver-related life-threatening outcomes – is critical for identifying this patient population in the clinical setting and improving the screening process of NASH clinical trials. We report the development and validation of NIS2+™, a diagnostic test designed as an optimisation of NIS4® technology, a blood-based panel currently used to detect at-risk NASH in patients with metabolic risk factors. NIS2+™ showed improved performance for the detection of at-risk NASH compared with NIS4® and other non-invasive liver tests that was not impacted by patients’ characteristics of interest, such as age, sex, type 2 diabetes mellitus, BMI, dyslipidaemia, and hypertension. This makes NIS2+™ a robust and reliable tool for the diagnosis of at-risk NASH among patients with metabolic risk factors, and an effective candidate for large-scale implementation in clinical practice and clinical trials.
中文翻译:

NIS2+™,基于血液的生物标志物 NIS4® 技术的优化,用于检测高危 NASH:一项前瞻性推导和验证研究
背景与目标
NIS4® 是一种基于血液的非侵入性检测,旨在有效排除/排除高危非酒精性脂肪性肝炎 (NASH),NASH 定义为非酒精性脂肪肝疾病活动评分≥4 且显着纤维化(≥2 期) ,存在代谢危险因素的患者。跨年龄、2 型糖尿病和性别等感兴趣特征的非侵入性测试分数的稳健性以及优化的分析方面对于临床实践中的大规模实施至关重要。我们开发并验证了 NIS2+™,它是 NIS4® 的优化版本,专门用于提高分数的稳健性。
方法
一个均衡的训练队列 (n = 198) 包括来自 GOLDEN-505 试验的患者。验证组 (n = 684) 和测试组 (n = 2,035) 包括来自 RESOLVE-IT 试验的患者。创建匹配良好的子组是为了避免在评分稳健性建模和分析过程中潜在的混杂效应。使用逻辑回归对模型进行训练,以进行高危 NASH 检测,并使用贝叶斯信息标准进行比较。使用受试者工作特征曲线下面积将 NIS2+™ 的性能与 NIS4®、Fibrosis-4 和丙氨酸转氨酶的性能进行比较,并通过评分分布分析稳健性。
结果
使用训练队列来比较 NIS4® 生物标志物的所有组合,NIS2(miR-34a-5p、YKL-40)被确定为最佳参数组合。为了纠正性别对 miR-34a-5p(验证队列)的影响,添加了性别和性别 * miR-34a-5p 参数,创建了 NIS2+™。在测试队列中,与NIS4®(0.792;p = 0.0002)、Fibrosis-4(0.653;p <0.0001)和丙氨酸转氨酶(0.699;p <0.0001)相比,NIS2+™ 在受试者操作特征曲线下面积 (0.813) 表现出统计上更高的面积。p <0.0001)。NIS2+™ 评分不受年龄、性别、BMI 或 2 型糖尿病状态的影响,无论患者特征如何,都能提供稳健的临床表现。
结论
NIS2+™ 是 NIS4® 技术的强大优化,用于检测高危 NASH。
影响和影响
开发非侵入性测试,用于准确、大规模检测高危非酒精性脂肪性肝炎(NASH;定义为非酒精性脂肪肝疾病活动评分≥4且纤维化阶段≥2的NASH)患者疾病进展和发生与肝脏相关的危及生命的结果的风险较高——对于在临床环境中识别这一患者群体并改进 NASH 临床试验的筛选过程至关重要。我们报告了 NIS2+™ 的开发和验证,这是一种诊断测试,旨在优化 NIS4® 技术,NIS4® 技术是一种基于血液的检测试剂盒,目前用于检测具有代谢危险因素的患者是否存在 NASH 风险。与 NIS4® 和其他非侵入性肝脏检测相比,NIS2+™ 在检测高危 NASH 方面表现出更高的性能,且不受患者感兴趣特征(如年龄、性别、2 型糖尿病、BMI、血脂异常、和高血压。这使得 NIS2+™ 成为诊断具有代谢危险因素的 NASH 患者的强大而可靠的工具,并且是在临床实践和临床试验中大规模实施的有效候选者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号