当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Radical-Triggered Annulation/Iododifluoromethylation of Enynones for the Stereospecific Synthesis of 1-Indenones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-05-23 , DOI: 10.1021/acs.joc.3c00471 Jia-Yin Wang 1 , Sai Zhang 2 , Qingkai Yuan 2 , Guigen Li 2 , Shenghu Yan 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-05-23 , DOI: 10.1021/acs.joc.3c00471 Jia-Yin Wang 1 , Sai Zhang 2 , Qingkai Yuan 2 , Guigen Li 2 , Shenghu Yan 1
Affiliation
|
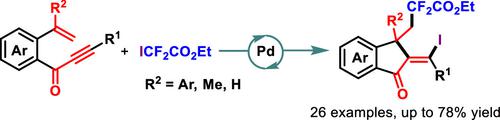
A new Pd(II)-catalyzed annulation/iododifluoromethylation of enynones has been developed for the synthesis of versatile 1-indanones with moderate to good yields (26 examples). The present strategy enabled the concomitant incorporation of two important difluoroalkyl and iodo functionalities into 1-indenone skeletons with (E)-stereoselectivity. The mechanistic pathway was proposed, consisting of the difluoroalkyl radical-triggered α,β-conjugated addition/5-exo-dig cyclization/metal radical cross-coupling/reductive elimination cascade.
中文翻译:

催化自由基引发的烯炔环化/碘二氟甲基化用于立体定向合成 1-茚酮
一种新的 Pd(II) 催化烯炔环化/碘二氟甲基化方法已被开发出来,用于合成具有中等至良好产率的多功能 1-茚满酮(26 个实例)。本策略能够将两个重要的二氟烷基和碘官能团同时并入具有 ( E )-立体选择性的 1-茚酮骨架中。提出了由二氟烷基自由基触发的α,β-共轭加成/5- exo -dig环化/金属自由基交叉偶联/还原消除级联组成的机理途径。
更新日期:2023-05-23
中文翻译:

催化自由基引发的烯炔环化/碘二氟甲基化用于立体定向合成 1-茚酮
一种新的 Pd(II) 催化烯炔环化/碘二氟甲基化方法已被开发出来,用于合成具有中等至良好产率的多功能 1-茚满酮(26 个实例)。本策略能够将两个重要的二氟烷基和碘官能团同时并入具有 ( E )-立体选择性的 1-茚酮骨架中。提出了由二氟烷基自由基触发的α,β-共轭加成/5- exo -dig环化/金属自由基交叉偶联/还原消除级联组成的机理途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号