当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scalable Atroposelective Synthesis of MRTX1719: An Inhibitor of the PRMT5/MTA Complex
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-05-10 , DOI: 10.1021/acs.oprd.3c00072 Michal Achmatowicz 1 , Thomas Scattolin 1 , David R. Snead 1 , Dinesh J. Paymode 1 , Sahar Roshandel 1 , Chen Xie 2 , Guihui Chen 3 , Cheng-yi Chen 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-05-10 , DOI: 10.1021/acs.oprd.3c00072 Michal Achmatowicz 1 , Thomas Scattolin 1 , David R. Snead 1 , Dinesh J. Paymode 1 , Sahar Roshandel 1 , Chen Xie 2 , Guihui Chen 3 , Cheng-yi Chen 1
Affiliation
|
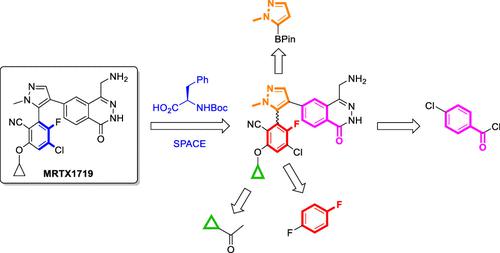
MRTX1719 was identified as a potent inhibitor of the PRMT5/MTA complex, designed to selectively target MTAP-deleted cancers. A scalable synthesis of this atropisomeric compound and an efficient isolation of the desired isomer were required to support Phase 1 clinical trials, and this was established through further development of the racemic medicinal chemistry route. In the key step, the desired (M)-atropisomer of MRTX1719 was amplified from racemic API by combining crystallization (20 °C) and racemization (160 °C, 4 min). Concurrent execution of these, ostensibly incompatible, operations was enabled by a continuous flow setup (SPACE = Simultaneous Processing of Antagonistic Chemical Events) providing 98.4% e.e. of (M)-atropisomer in 75% yield from racemic API on 12 kg scale. Process development targeting earlier steps of the API synthesis led to several impactful revisions including desymmetrization of 4-chlorobenzamide to access the 6-substituted-4-(aminomethyl)phthalazin-1(2H)-one ring system, improved borylation conditions (Suzuki–Miyaura or photocatalytic), and demonstration of an economically viable route to the challenging pentasubstituted benzene from 1,4-difluorobenzene and cyclopropyl methyl ketone.
中文翻译:

MRTX1719 的可扩展阻转选择性合成:PRMT5/MTA 复合物的抑制剂
MRTX1719被鉴定为 PRMT5/MTA 复合物的有效抑制剂,旨在选择性地靶向MTAP缺失的癌症。这种阻转异构化合物的可扩展合成和所需异构体的有效分离需要支持 1 期临床试验,这是通过外消旋药物化学途径的进一步开发建立的。在关键步骤中,通过结合结晶 (20 °C) 和外消旋化(160 °C,4 分钟),从外消旋 API 中扩增出所需的MRTX1719 ( M )-atropoisomer 。这些表面上不兼容的操作的并发执行是通过连续流设置实现的(SPACE = S imultaneous Processing of A拮抗剂C化学E孔)在 12 千克规模的外消旋 API 中以 75% 的产率提供 98.4% ee 的 ( M )-阻转异构体。针对 API 合成早期步骤的工艺开发导致了一些有影响的修订,包括 4-氯苯甲酰胺的去对称化以获得 6-取代的-4-(氨基甲基)酞嗪-1(2 H)-一环系统,改进的硼酸化条件( Suzuki- Miyaura 或光催化),并展示了从 1,4-二氟苯和环丙基甲基酮到具有挑战性的五取代苯的经济可行路线。
更新日期:2023-05-10
中文翻译:

MRTX1719 的可扩展阻转选择性合成:PRMT5/MTA 复合物的抑制剂
MRTX1719被鉴定为 PRMT5/MTA 复合物的有效抑制剂,旨在选择性地靶向MTAP缺失的癌症。这种阻转异构化合物的可扩展合成和所需异构体的有效分离需要支持 1 期临床试验,这是通过外消旋药物化学途径的进一步开发建立的。在关键步骤中,通过结合结晶 (20 °C) 和外消旋化(160 °C,4 分钟),从外消旋 API 中扩增出所需的MRTX1719 ( M )-atropoisomer 。这些表面上不兼容的操作的并发执行是通过连续流设置实现的(SPACE = S imultaneous Processing of A拮抗剂C化学E孔)在 12 千克规模的外消旋 API 中以 75% 的产率提供 98.4% ee 的 ( M )-阻转异构体。针对 API 合成早期步骤的工艺开发导致了一些有影响的修订,包括 4-氯苯甲酰胺的去对称化以获得 6-取代的-4-(氨基甲基)酞嗪-1(2 H)-一环系统,改进的硼酸化条件( Suzuki- Miyaura 或光催化),并展示了从 1,4-二氟苯和环丙基甲基酮到具有挑战性的五取代苯的经济可行路线。


























 京公网安备 11010802027423号
京公网安备 11010802027423号