Cell ( IF 64.5 ) Pub Date : 2023-04-24 , DOI: 10.1016/j.cell.2023.03.030 Yanyan Wang 1 , Senxin Zhang 2 , Xinrui Yang 3 , Joyce K Hwang 4 , Chuanzong Zhan 5 , Chaoyang Lian 5 , Chong Wang 4 , Tuantuan Gui 5 , Binbin Wang 5 , Xia Xie 6 , Pengfei Dai 6 , Lu Zhang 7 , Ying Tian 5 , Huizhi Zhang 8 , Chong Han 6 , Yanni Cai 6 , Qian Hao 5 , Xiaofei Ye 9 , Xiaojing Liu 6 , Jiaquan Liu 6 , Zhiwei Cao 7 , Shaohui Huang 10 , Jie Song 11 , Qiang Pan-Hammarström 12 , Yaofeng Zhao 13 , Frederick W Alt 4 , Xiaoqi Zheng 14 , Lin-Tai Da 3 , Leng-Siew Yeap 15 , Fei-Long Meng 16
|
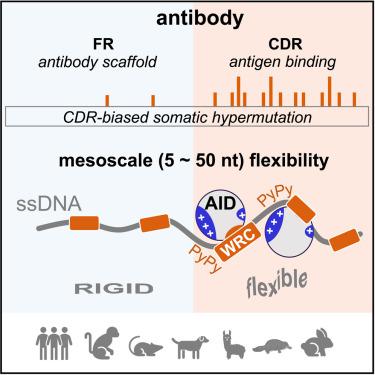
Somatic hypermutation (SHM), initiated by activation-induced cytidine deaminase (AID), generates mutations in the antibody-coding sequence to allow affinity maturation. Why these mutations intrinsically focus on the three nonconsecutive complementarity-determining regions (CDRs) remains enigmatic. Here, we found that predisposition mutagenesis depends on the single-strand (ss) DNA substrate flexibility determined by the mesoscale sequence surrounding AID deaminase motifs. Mesoscale DNA sequences containing flexible pyrimidine-pyrimidine bases bind effectively to the positively charged surface patches of AID, resulting in preferential deamination activities. The CDR hypermutability is mimicable in in vitro deaminase assays and is evolutionarily conserved among species using SHM as a major diversification strategy. We demonstrated that mesoscale sequence alterations tune the in vivo mutability and promote mutations in an otherwise cold region in mice. Our results show a non-coding role of antibody-coding sequence in directing hypermutation, paving the way for the synthetic design of humanized animal models for optimal antibody discovery and explaining the AID mutagenesis pattern in lymphoma.
中文翻译:

抗体编码序列中的中尺度 DNA 特征促进体细胞超突变
由激活诱导的胞苷脱氨酶 (AID) 引发的体细胞超突变 (SHM) 在抗体编码序列中产生突变,以实现亲和力成熟。为什么这些突变本质上集中在三个不连续的互补决定区(CDR)仍然是个谜。在这里,我们发现易感诱变取决于单链 (ss) DNA 底物的灵活性,而单链 DNA 底物的灵活性是由 AID 脱氨酶基序周围的中尺度序列决定的。含有柔性嘧啶-嘧啶碱基的中尺度DNA序列有效地结合到AID带正电荷的表面斑块上,从而产生优先的脱氨活性。CDR 超突变性在体外脱氨酶测定中是可模拟的,并且在使用 SHM 作为主要多样化策略的物种之间进化上是保守的。我们证明,中尺度序列改变可以调节体内突变性,并促进小鼠寒冷区域的突变。我们的结果显示了抗体编码序列在指导超突变中的非编码作用,为人源化动物模型的合成设计以实现最佳抗体发现铺平了道路,并解释了淋巴瘤中的 AID 突变模式。


























 京公网安备 11010802027423号
京公网安备 11010802027423号