Resources, Conservation and Recycling ( IF 13.2 ) Pub Date : 2023-04-23 , DOI: 10.1016/j.resconrec.2023.107004 Shiyu Li, Thien Q. Tran, Qi Li, Bin Ji, Alexander S. Brand, Wencai Zhang
|
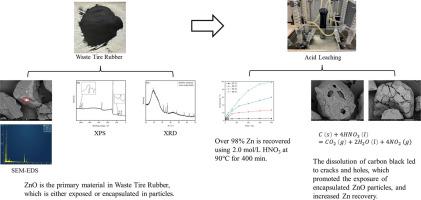
Experiments were conducted to investigate the leaching behavior of Zn from end-of-life tire rubber. XRD and XPS analyses showed that the Zn-containing phase in the material was mainly ZnO. SEM-EDS analysis confirmed the presence of both exposed and encapsulated ZnO particles. Acid leaching tests indicated that the carbon black comprised in the material can be oxidized and dissolved by HNO3, resulting in the dissolution of completely encapsulated ZnO particles with thinner covering layers. Then, leaching tests using HNO3 as the lixiviant were performed by varying acid concentration and leaching temperature. The results showed over 98% of Zn can be recovered using 2.0 mol/L HNO3 at 90 ℃ after 400 min of reaction. Leaching kinetic results were best fit with the Avrami model, indicating the leaching process was controlled by diffusion. The activation energy determined by Arrhenius formula was 12.92 kJ/mol, which further supports the proposed diffusion controlled leaching process.
中文翻译:

报废轮胎橡胶中锌的浸出回收和机制
进行了实验以研究报废轮胎橡胶中锌的浸出行为。XRD和XPS分析表明材料中的含Zn相主要为ZnO。SEM-EDS 分析证实了暴露的和封装的 ZnO 颗粒的存在。酸浸试验表明材料中的炭黑可以被HNO 3氧化溶解,导致完全包封的ZnO颗粒溶解,覆盖层较薄。然后,通过改变酸浓度和浸出温度,以HNO 3作为浸出剂进行浸出试验。结果表明,使用 2.0 mol/L HNO 3可以回收 98% 以上的 Zn90℃反应400分钟后。浸出动力学结果与 Avrami 模型最吻合,表明浸出过程受扩散控制。Arrhenius 公式确定的活化能为 12.92 kJ/mol,这进一步支持了所提出的扩散控制浸出过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号