当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Concise Process for the Synthesis of the Azaindazole Core of the CD73 Inhibitor AB680
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-04-19 , DOI: 10.1021/acs.oprd.3c00056 Xiaopeng Yin 1 , Zhiguo Jake Song 1 , Jaroslaw Kalisiak 1 , Jonathan C. Tripp 2 , Xiao Lei 3 , Hongfeng Li 3 , Aimin Zhang 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-04-19 , DOI: 10.1021/acs.oprd.3c00056 Xiaopeng Yin 1 , Zhiguo Jake Song 1 , Jaroslaw Kalisiak 1 , Jonathan C. Tripp 2 , Xiao Lei 3 , Hongfeng Li 3 , Aimin Zhang 1
Affiliation
|
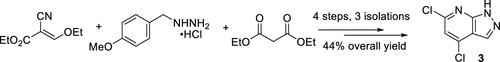
AB680 is a highly potent small-molecule CD73 inhibitor discovered and developed by Arcus Biosciences, currently in clinical trials for the treatment of pancreatic cancer. Herein, we report a concise synthesis of 4,6-dichloro-1H-pyrazolo[3,4-b]pyridine 3, which is the central azaindazole core of AB680. The process consists of four synthetic operations and three isolations, including a PMB-protected pyrazole formation, a telescoped two-step cyclization and aromatization sequence, and finally a one-pot PMB deprotection and chlorination to furnish 3. This chemistry was successfully scaled up to provide >200 g of 3 in 44% overall yield and 97.6% HPLC purity. Overall, the route developed toward 3 represents an efficient construction of an azaindazole core, which is a synthetically challenging yet prevalent structural motif.
中文翻译:

开发用于合成 CD73 抑制剂 AB680 的氮杂吲唑核心的简明工艺
AB680是Arcus Biosciences发现和开发的一种高效小分子CD73抑制剂,目前正处于治疗胰腺癌的临床试验阶段。在此,我们报告了 4,6-二氯-1H-吡唑并 [3,4- b ] 吡啶3的简明合成,它是 AB680 的中心氮杂吲唑核心。该过程包括四个合成操作和三个分离,包括 PMB 保护的吡唑形成、伸缩的两步环化和芳构化序列,以及最后的一锅 PMB 脱保护和氯化以提供3。该化学反应成功放大,可提供 >200 g 的3,总收率为 44%,HPLC 纯度为 97.6%。总体而言,路线向3代表了氮杂吲唑核的有效构建,这是一种具有综合挑战性但普遍存在的结构基序。
更新日期:2023-04-19
中文翻译:

开发用于合成 CD73 抑制剂 AB680 的氮杂吲唑核心的简明工艺
AB680是Arcus Biosciences发现和开发的一种高效小分子CD73抑制剂,目前正处于治疗胰腺癌的临床试验阶段。在此,我们报告了 4,6-二氯-1H-吡唑并 [3,4- b ] 吡啶3的简明合成,它是 AB680 的中心氮杂吲唑核心。该过程包括四个合成操作和三个分离,包括 PMB 保护的吡唑形成、伸缩的两步环化和芳构化序列,以及最后的一锅 PMB 脱保护和氯化以提供3。该化学反应成功放大,可提供 >200 g 的3,总收率为 44%,HPLC 纯度为 97.6%。总体而言,路线向3代表了氮杂吲唑核的有效构建,这是一种具有综合挑战性但普遍存在的结构基序。


























 京公网安备 11010802027423号
京公网安备 11010802027423号