当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electron Transfer Energy and Hydrogen Atom Transfer Energy-Based Linear Free Energy Relationships for Predicting the Rate Constants of Munition Constituent Reduction by Hydroquinones
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.est.2c08931 Jimmy Murillo-Gelvez 1 , Kevin Hickey 1 , Dominic M Di Toro 1 , Herbert E Allen 1 , Richard F Carbonaro 2, 3 , Pei C Chiu 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.est.2c08931 Jimmy Murillo-Gelvez 1 , Kevin Hickey 1 , Dominic M Di Toro 1 , Herbert E Allen 1 , Richard F Carbonaro 2, 3 , Pei C Chiu 1
Affiliation
|
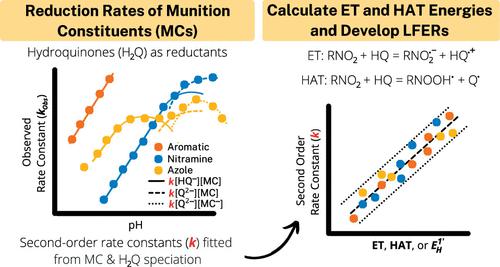
No single linear free energy relationship (LFER) exists that can predict reduction rate constants of all munition constituents (MCs). To address this knowledge gap, we measured the reduction rates of MCs and their surrogates including nitroaromatics [NACs; 2,4,6-trinitrotoluene (TNT), 2,4-dinitroanisole (DNAN), 2-amino-4,6-dinitrotoluene (2-A-DNT), 4-amino-2,6-dinitrotoluene (4-A-DNT), and 2,4-dinitrotoluene (DNT)], nitramines [hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and nitroguanidine (NQ)], and azoles [3-nitro-1,2,4-triazol-5-one (NTO) and 3,4-dinitropyrazole (DNP)] by three dithionite-reduced quinones (lawsone, AQDS, and AQS). All MCs/NACs were reduced by the hydroquinones except NQ. Hydroquinone and MC speciations were varied by controlling pH, permitting the application of a speciation model to determine second-order rate constants (k) from observed pseudo-first-order rate constants. The intrinsic reactivity of MCs (oxidants) decreased upon deprotonation, while the opposite was true for hydroquinones (reductants). The rate constants spanned ∼6 orders of magnitude in the order NTO ≈ TNT > DNP > DNT ≈ DNAN ≈ 2-A-DNT > DNP– > 4-A-DNT > NTO– > RDX. LFERs developed using density functional theory-calculated electron transfer and hydrogen atom transfer energies and reported one-electron reduction potentials successfully predicted k, suggesting that these structurally diverse MCs/NACs are all reduced by hydroquinones through the same mechanism and rate-limiting step. These results increase the applicability of LFER models for predicting the fate and half-lives of MCs and related nitro compounds in reducing environments.
中文翻译:

基于电子转移能和氢原子转移能的线性自由能关系预测对苯二酚对军火成分还原的速率常数
不存在可以预测所有弹药成分 (MC) 的还原率常数的单一线性自由能关系 (LFER)。为了解决这一知识差距,我们测量了 MC 及其替代物(包括硝基芳烃 [NACs;2,4,6-三硝基甲苯 (TNT)、2,4-二硝基苯甲醚 (DNAN)、2-氨基-4,6-二硝基甲苯 (2-A-DNT)、4-氨基-2,6-二硝基甲苯 (4-A -DNT)、2,4-二硝基甲苯 (DNT)]、硝胺 [六氢-1,3,5-三硝基-1,3,5-三嗪 (RDX) 和硝基胍 (NQ)],以及唑类 [3-硝基-1,2,4-三唑-5-酮 (NTO) 和 3,4-二硝基吡唑 (DNP)] 由三种连二亚硫酸盐还原的醌类(lawsone、AQDS 和 AQS)合成。除 NQ 外,所有 MC/NAC 都被对苯二酚还原。通过控制 pH 来改变对苯二酚和 MC 的形态,从而允许应用形态模型来确定二阶速率常数(k ) 来自观察到的伪一级速率常数。MCs(氧化剂)的固有反应性在去质子化时降低,而对苯二酚(还原剂)则相反。速率常数按 NTO ≈ TNT > DNP > DNT ≈ DNAN ≈ 2-A-DNT > DNP – > 4-A-DNT > NTO – > RDX 的顺序跨越了约 6 个数量级。使用密度泛函理论开发的 LFER——计算电子转移和氢原子转移能量并报告单电子还原电势成功预测k,表明这些结构不同的 MCs/NACs 都通过相同的机制和限速步骤被对苯二酚还原。这些结果增加了 LFER 模型在预测 MC 和相关硝基化合物在还原环境中的归宿和半衰期方面的适用性。
更新日期:2023-03-24
中文翻译:

基于电子转移能和氢原子转移能的线性自由能关系预测对苯二酚对军火成分还原的速率常数
不存在可以预测所有弹药成分 (MC) 的还原率常数的单一线性自由能关系 (LFER)。为了解决这一知识差距,我们测量了 MC 及其替代物(包括硝基芳烃 [NACs;2,4,6-三硝基甲苯 (TNT)、2,4-二硝基苯甲醚 (DNAN)、2-氨基-4,6-二硝基甲苯 (2-A-DNT)、4-氨基-2,6-二硝基甲苯 (4-A -DNT)、2,4-二硝基甲苯 (DNT)]、硝胺 [六氢-1,3,5-三硝基-1,3,5-三嗪 (RDX) 和硝基胍 (NQ)],以及唑类 [3-硝基-1,2,4-三唑-5-酮 (NTO) 和 3,4-二硝基吡唑 (DNP)] 由三种连二亚硫酸盐还原的醌类(lawsone、AQDS 和 AQS)合成。除 NQ 外,所有 MC/NAC 都被对苯二酚还原。通过控制 pH 来改变对苯二酚和 MC 的形态,从而允许应用形态模型来确定二阶速率常数(k ) 来自观察到的伪一级速率常数。MCs(氧化剂)的固有反应性在去质子化时降低,而对苯二酚(还原剂)则相反。速率常数按 NTO ≈ TNT > DNP > DNT ≈ DNAN ≈ 2-A-DNT > DNP – > 4-A-DNT > NTO – > RDX 的顺序跨越了约 6 个数量级。使用密度泛函理论开发的 LFER——计算电子转移和氢原子转移能量并报告单电子还原电势成功预测k,表明这些结构不同的 MCs/NACs 都通过相同的机制和限速步骤被对苯二酚还原。这些结果增加了 LFER 模型在预测 MC 和相关硝基化合物在还原环境中的归宿和半衰期方面的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号