Structure ( IF 5.7 ) Pub Date : 2023-03-23 , DOI: 10.1016/j.str.2023.02.014 Dmitry S Karlov 1 , Sarah L Long 2 , Ximin Zeng 3 , Fuzhou Xu 4 , Kanhaya Lal 1 , Liu Cao 3 , Karim Hayoun 2 , Jun Lin 3 , Susan A Joyce 2 , Irina G Tikhonova 1
|
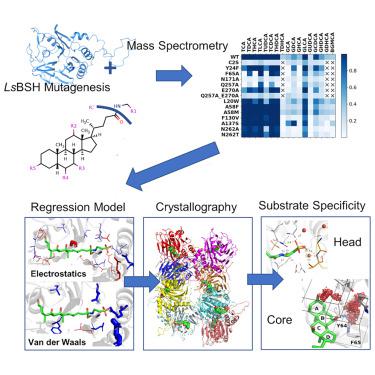
Bile salt hydrolases (BSHs) are currently being investigated as target enzymes for metabolic regulators in humans and as growth promoters in farm animals. Understanding structural features underlying substrate specificity is necessary for inhibitor design. Here, we used a multidisciplinary workflow including mass spectrometry, mutagenesis, molecular dynamic simulations, machine learning, and crystallography to demonstrate substrate specificity in Lactobacillus salivarius BSH, the most abundant enzyme in human and farm animal intestines. We show the preference of substrates with a taurine head and a dehydroxylated sterol ring for hydrolysis. A regression model that correlates the relative rates of hydrolysis of various substrates in various enzyme mutants with the residue-substrate interaction energies guided the identification of structural determinants of substrate binding and specificity. In addition, we found T208 from another BSH protomer regulating the hydrolysis. The designed workflow can be used for fast and comprehensive characterization of enzymes with a broad range of substrates.
中文翻译:

通过实验和计算分析表征胆盐水解酶底物特异性的机制
目前正在研究胆盐水解酶 (BSH) 作为人类代谢调节剂的靶酶和农场动物的生长促进剂。了解底物特异性的结构特征对于抑制剂设计是必要的。在这里,我们使用了包括质谱、诱变、分子动力学模拟、机器学习和晶体学在内的多学科工作流程来证明唾液乳杆菌的底物特异性BSH,人类和农场动物肠道中最丰富的酶。我们显示了具有牛磺酸头和脱羟基甾醇环的底物对水解的偏好。将各种酶突变体中各种底物的相对水解速率与残基-底物相互作用能相关联的回归模型指导了底物结合和特异性的结构决定因素的鉴定。此外,我们还从另一个调节水解的 BSH 原体中发现了 T208。设计的工作流程可用于对具有广泛底物的酶进行快速和全面的表征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号