当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MnO2-Induced Oxidation of Iodide in Frozen Solution
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.est.3c00604 Juanshan Du 1 , Kitae Kim 2 , Seungwoo Son 3 , Donglai Pan 4 , Sunghwan Kim 3 , Wonyong Choi 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.est.3c00604 Juanshan Du 1 , Kitae Kim 2 , Seungwoo Son 3 , Donglai Pan 4 , Sunghwan Kim 3 , Wonyong Choi 1
Affiliation
|
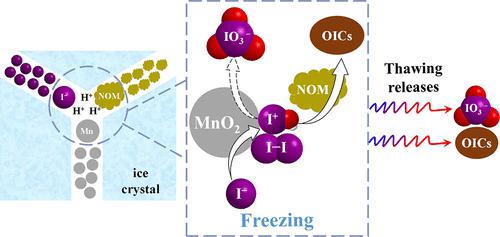
Metal oxides play a critical role in the abiotic transformation of iodine species in natural environments. In this study, we investigated iodide oxidation by manganese dioxides (β-MnO2, γ-MnO2, and δ-MnO2) in frozen and aqueous solutions. The heterogeneous reaction produced reactive iodine (RI) in the frozen phase, and the subsequent thawing of the frozen sample induced the gradual transformation of in situ-formed RI to iodate or iodide, depending on the types of manganese dioxides. The freezing-enhanced production of RI was observed over the pH range of 5.0–9.0, but it decreased with increasing pH. Fulvic acid (FA) can be iodinated by I–/MnO2 in aqueous and frozen solutions. About 0.8–8.4% of iodide was transformed to organoiodine compounds (OICs) at pH 6.0–7.8 in aqueous solution, while higher yields (10.4–17.8%) of OICs were obtained in frozen solution. Most OICs generated in the frozen phase contained one iodine atom and were lignin-like compounds according to Fourier transform ion cyclotron resonance/mass spectrometry analysis. This study uncovers a previously unrecognized production pathway of OICs under neutral conditions in frozen environments.
中文翻译:

MnO2 诱导冷冻溶液中碘化物的氧化
金属氧化物在自然环境中碘物种的非生物转化中起着关键作用。在这项研究中,我们研究了二氧化锰(β-MnO 2、γ-MnO 2和 δ-MnO 2)在冷冻溶液和水溶液中对碘化物的氧化作用。多相反应在冷冻相中产生活性碘 (RI),随后冷冻样品解冻会导致原位形成的 RI 逐渐转化为碘酸盐或碘化物,具体取决于二氧化锰的类型。在 5.0-9.0 的 pH 范围内观察到冷冻增强的 RI 产生,但它随着 pH 的增加而降低。富里酸 (FA) 可以被 I – /MnO 2碘化在水溶液和冷冻溶液中。在 pH 6.0-7.8 的水溶液中,大约 0.8-8.4% 的碘化物转化为有机碘化合物 (OIC),而在冷冻溶液中获得更高产率 (10.4-17.8%) 的 OIC。根据傅立叶变换离子回旋共振/质谱分析,在冷冻相中产生的大多数 OIC 含有一个碘原子,并且是木质素样化合物。这项研究揭示了在冷冻环境中性条件下以前未被认识的 OICs 生产途径。
更新日期:2023-03-23
中文翻译:

MnO2 诱导冷冻溶液中碘化物的氧化
金属氧化物在自然环境中碘物种的非生物转化中起着关键作用。在这项研究中,我们研究了二氧化锰(β-MnO 2、γ-MnO 2和 δ-MnO 2)在冷冻溶液和水溶液中对碘化物的氧化作用。多相反应在冷冻相中产生活性碘 (RI),随后冷冻样品解冻会导致原位形成的 RI 逐渐转化为碘酸盐或碘化物,具体取决于二氧化锰的类型。在 5.0-9.0 的 pH 范围内观察到冷冻增强的 RI 产生,但它随着 pH 的增加而降低。富里酸 (FA) 可以被 I – /MnO 2碘化在水溶液和冷冻溶液中。在 pH 6.0-7.8 的水溶液中,大约 0.8-8.4% 的碘化物转化为有机碘化合物 (OIC),而在冷冻溶液中获得更高产率 (10.4-17.8%) 的 OIC。根据傅立叶变换离子回旋共振/质谱分析,在冷冻相中产生的大多数 OIC 含有一个碘原子,并且是木质素样化合物。这项研究揭示了在冷冻环境中性条件下以前未被认识的 OICs 生产途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号