Phytomedicine ( IF 7.9 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.phymed.2023.154783 Xuemei Liu 1 , Yan Yu 2 , Yanqing Wu 2 , Ai Luo 1 , Mei Yang 2 , Ting Li 2 , Tingqian Li 2 , Bing Mao 2 , Xiaoting Chen 3 , Juanjuan Fu 2 , Hongli Jiang 2 , Wei Liu 1
|
Background
The clinical effect of Yupingfeng (YPF) has been confirmed in asthma patients, however, it lacks a study to verify its pharmacological mechanism.
Hypothesis/purpose
To reveal the molecular basis and potential pharmacological mechanism of YPF in the treatment of asthma.
Study design and methods
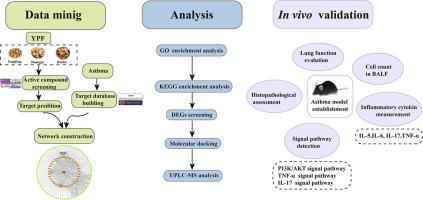
First, a systems pharmacology-based method integrating pharmacokinetic screening, target prediction, network analyses, GO and KEGG analyses were used for the systematic deciphering of the mechanism of YPF in asthma. Second, differentially expressed genes (DEGs) between asthma patients and healthy controls were identified by GEO2R online tool. Third, based on systems pharmacology and DEGs results, molecular docking was performed utilizing the Discovery Studio 2020 Client version to detect the binding capacity between compounds and targets. Finally, ovalbumin (OVA)-challenged C57BL/6 mice were treated with YPF or its effective compound to assess the predictions.
Results
A total of 35 active compounds were filtered out, with 87 potential targets being identified for further analysis after target fishing and matching. Quercetin, kaempferol, and wogonin were identified as the main ingredients in YPF. The signaling pathways of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), tumor necrosis factor (TNF) and IL-17 were identified as the top signaling pathways in KEGG enrichment analysis. GEO2R tools of NCBI discovered five DEGs that overlapped with the therapeutic targets of YPF. Wogonin was proven to be the top active compound in YPF through the results of molecular docking. In vivo experiments indicated that YPF and wogonin significantly attenuated airway resistance and lung inflammation by decreasing the levels of inflammatory cytokines and key factors in PI3K/AKT, IL-17, and TNF signaling pathways.
Conclusions
YPF and its main active compound wogonin may exert some therapeutic effects on asthma inflammation through multiple molecular targets and signaling pathways including PI3K/AKT, IL-17 and TNF-α.
中文翻译:

基于系统药理学的体内研究揭示玉屏风治疗哮喘的有效机制
背景
玉屏风(YPF)在哮喘患者中的临床疗效已得到证实,但缺乏研究验证其药理机制。
假设/目的
揭示YPF治疗哮喘的分子基础和潜在药理机制。
研究设计和方法
首先,采用基于系统药理学的药代动力学筛选、靶点预测、网络分析、GO 和 KEGG 分析相结合的方法系统地破译 YPF 在哮喘中的作用机制。其次,通过 GEO2R 在线工具识别哮喘患者和健康对照之间的差异表达基因 (DEG)。第三,基于系统药理学和DEGs结果,利用Discovery Studio 2020 Client版本进行分子对接,检测化合物与靶点的结合能力。最后,用 YPF 或其有效化合物处理卵清蛋白 (OVA) 攻击的 C57BL/6 小鼠以评估预测。
结果
共筛选出 35 种活性化合物,经过靶点捕捞和匹配后,确定了 87 个潜在靶点用于进一步分析。槲皮素、山柰酚和汉黄芩素被确定为 YPF 中的主要成分。磷脂酰肌醇 3-激酶 (PI3K)/蛋白激酶 B (AKT)、肿瘤坏死因子 (TNF) 和 IL-17 的信号通路被确定为 KEGG 富集分析中的顶级信号通路。NCBI 的 GEO2R 工具发现了五个与 YPF 的治疗靶点重叠的 DEG。通过分子对接结果证明汉黄芩素是YPF中活性最高的化合物。体内实验表明,YPF 和汉黄芩素通过降低炎症细胞因子水平和 PI3K/AKT、IL-17 和 TNF 信号通路中的关键因子水平,显着减轻气道阻力和肺部炎症。
结论
YPF及其主要活性成分汉黄芩素可能通过PI3K/AKT、IL-17、TNF-α等多个分子靶点和信号通路对哮喘炎症发挥一定的治疗作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号