Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2023-03-20 , DOI: 10.1016/j.cej.2023.142510 Yongxiao Tuo , Wanli Liu , Qing Lu , Xingzhao Wang , Jiabing Luo , Shutao Wang , Yan Zhou , Min Wang , Xiaohui Sun , Xiang Feng , Mingbo Wu , De Chen , Jun Zhang
|
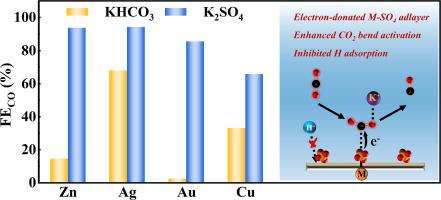
The most difficulty of efficient CO2 electroreduction lies in the activation step that turns CO2 into the CO2 − radicals or other intermediates that can be further converted. To overcome this bottleneck, many efforts have been devoted to develop electrocatalysts to lower the overpotential of CO2 activation, but they inevitably involve complicated or/and unscalable synthesis methods. Here we reported that the SO42− ion modified metal foils, including Zn, Ag, Au and Cu, demonstrated ∼6.5, 1.4, 14 and 2.5 times of CO faradaic efficiency in K2SO4 electrolyte compared to KHCO3 electrolyte at −1.05 V vs. RHE. According to both experimental and DFT studies, the electron-rich metal-SO4 adlayer can activate CO2 by bending the linear molecule of CO2 through donating electrons to the antibonding orbital of CO2, thus facilitating the formation of key intermediate *CO2
− radicals or other intermediates that can be further converted. To overcome this bottleneck, many efforts have been devoted to develop electrocatalysts to lower the overpotential of CO2 activation, but they inevitably involve complicated or/and unscalable synthesis methods. Here we reported that the SO42− ion modified metal foils, including Zn, Ag, Au and Cu, demonstrated ∼6.5, 1.4, 14 and 2.5 times of CO faradaic efficiency in K2SO4 electrolyte compared to KHCO3 electrolyte at −1.05 V vs. RHE. According to both experimental and DFT studies, the electron-rich metal-SO4 adlayer can activate CO2 by bending the linear molecule of CO2 through donating electrons to the antibonding orbital of CO2, thus facilitating the formation of key intermediate *CO2 −. Moreover, the adsorption of H+ on metal sites is restrained by the metal-SO4 adlayer, leading to a suppressed H2 evolution activity. From a broader perspective, many catalysts can benefit from this approach to achieve more efficient CO2 electrochemical reduction.
−. Moreover, the adsorption of H+ on metal sites is restrained by the metal-SO4 adlayer, leading to a suppressed H2 evolution activity. From a broader perspective, many catalysts can benefit from this approach to achieve more efficient CO2 electrochemical reduction.
中文翻译:

SO42− 介导金属电极上的 CO2 活化以实现高效的 CO2 电还原
有效CO 2电还原的最大困难在于将CO 2转化为CO 2  -自由基或其他可以进一步转化的中间体的活化步骤。为了克服这一瓶颈,许多努力致力于开发电催化剂以降低 CO 2活化的过电势,但它们不可避免地涉及复杂或/和不可扩展的合成方法。在这里,我们报道了 SO 4 2-离子改性金属箔(包括 Zn、Ag、Au 和 Cu)在 K 2 SO 4电解质中的 CO 法拉第效率是 KHCO 3的 ~6.5、1.4、14 和 2.5 倍电解质为 -1.05 V vs. RHE。根据实验和DFT研究,富电子金属-SO 4吸附层可以通过向CO 2 的反键轨道提供电子来弯曲CO 2的线性分子来激活CO 2 ,从而促进关键中间体* CO 2的形成-。此外,H +在金属位点上的吸附受到金属-SO 4吸附层的限制,导致H 2释放活性受到抑制。从更广泛的角度来看,许多催化剂可以从这种方法中受益,以实现更高效的 CO 2电化学还原。
-自由基或其他可以进一步转化的中间体的活化步骤。为了克服这一瓶颈,许多努力致力于开发电催化剂以降低 CO 2活化的过电势,但它们不可避免地涉及复杂或/和不可扩展的合成方法。在这里,我们报道了 SO 4 2-离子改性金属箔(包括 Zn、Ag、Au 和 Cu)在 K 2 SO 4电解质中的 CO 法拉第效率是 KHCO 3的 ~6.5、1.4、14 和 2.5 倍电解质为 -1.05 V vs. RHE。根据实验和DFT研究,富电子金属-SO 4吸附层可以通过向CO 2 的反键轨道提供电子来弯曲CO 2的线性分子来激活CO 2 ,从而促进关键中间体* CO 2的形成-。此外,H +在金属位点上的吸附受到金属-SO 4吸附层的限制,导致H 2释放活性受到抑制。从更广泛的角度来看,许多催化剂可以从这种方法中受益,以实现更高效的 CO 2电化学还原。


























 京公网安备 11010802027423号
京公网安备 11010802027423号