当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neglected but Efficient Electron Utilization Driven by Biochar-Coactivated Phenols and Peroxydisulfate: Polyphenol Accumulation Rather than Mineralization
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-18 , DOI: 10.1021/acs.est.3c00022 Jibo Dou 1 , Yao Tang 1 , Zhijiang Lu 2 , Guangzhi He 3 , Jianming Xu 1 , Yan He 1, 4
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2023-03-18 , DOI: 10.1021/acs.est.3c00022 Jibo Dou 1 , Yao Tang 1 , Zhijiang Lu 2 , Guangzhi He 3 , Jianming Xu 1 , Yan He 1, 4
Affiliation
|
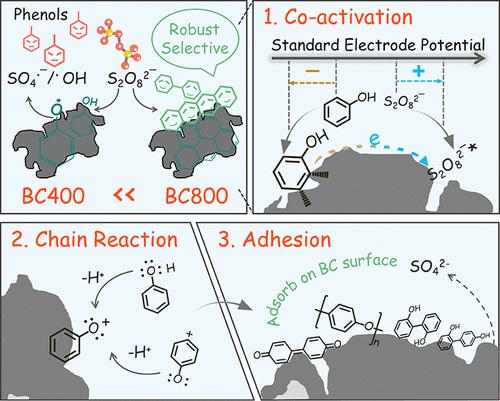
We report an unrecognized but efficient nonradical mechanism in biochar-activated peroxydisulfate (PDS) systems. Combining a newly developed fluorescence trapper of reactive oxygen species with steady-state concentration calculations, we showed that raising pyrolysis temperatures of biochar (BC) from 400 to 800 °C remarkably enhanced trichlorophenol degradation but inhibited the catalytic production of radicals (SO4•– and •OH) in water and soil, thereby switching a radical-based activation into an electron-transfer-dominated nonradical pathway (contribution increased from 12.9 to 76.9%). Distinct from previously reported PDS* complex-determined oxidation, in situ Raman and electrochemical results of this study demonstrated that the simultaneous activation of phenols and PDS on the biochar surface triggers the potential difference-driven electron transfer. The formed phenoxy radicals subsequently undergo coupling and polymerization reactions to generate dimeric and oligomeric intermediates, which are eventually accumulated on the biochar surface and removed. Such a unique nonmineralizing oxidation achieved an ultrahigh electron utilization efficiency (ephenols/ePDS) of 182%. Through biochar molecular modeling and theoretical calculations, we highlighted the critical role of graphitic domains rather than redox-active moieties in lowering band-gap energy to facilitate electron transfer. Our work provides insights into outstanding contradictions and controversies related to nonradical oxidation and inspiration for more oxidant-saving remediation technologies.
中文翻译:

生物炭共活化酚和过二硫酸盐驱动的被忽视但有效的电子利用:多酚积累而不是矿化
我们报告了生物炭激活的过二硫酸盐 (PDS) 系统中一种未被识别但有效的非自由基机制。结合新开发的活性氧荧光捕集器和稳态浓度计算,我们表明将生物炭 (BC) 的热解温度从 400 °C 提高到 800 °C 显着增强了三氯苯酚的降解,但抑制了自由基 (SO 4 • – • OH) 在水和土壤中,从而将基于自由基的激活转变为电子转移主导的非自由基途径(贡献从 12.9 %增加到 76.9%)。不同于之前报道的 PDS* 复杂原位氧化该研究的拉曼和电化学结果表明,生物炭表面酚类和 PDS 的同时活化触发了电位差驱动的电子转移。形成的苯氧基自由基随后进行偶联和聚合反应,生成二聚和低聚中间体,这些中间体最终积聚在生物炭表面并被去除。这种独特的非矿化氧化实现了超高的电子利用效率(e phenols / e PDS) 的 182%。通过生物炭分子建模和理论计算,我们强调了石墨域而非氧化还原活性部分在降低带隙能量以促进电子转移方面的关键作用。我们的工作提供了对与非自由基氧化相关的突出矛盾和争议的见解,并为更多节省氧化剂的修复技术提供了灵感。
更新日期:2023-03-18
中文翻译:

生物炭共活化酚和过二硫酸盐驱动的被忽视但有效的电子利用:多酚积累而不是矿化
我们报告了生物炭激活的过二硫酸盐 (PDS) 系统中一种未被识别但有效的非自由基机制。结合新开发的活性氧荧光捕集器和稳态浓度计算,我们表明将生物炭 (BC) 的热解温度从 400 °C 提高到 800 °C 显着增强了三氯苯酚的降解,但抑制了自由基 (SO 4 • – • OH) 在水和土壤中,从而将基于自由基的激活转变为电子转移主导的非自由基途径(贡献从 12.9 %增加到 76.9%)。不同于之前报道的 PDS* 复杂原位氧化该研究的拉曼和电化学结果表明,生物炭表面酚类和 PDS 的同时活化触发了电位差驱动的电子转移。形成的苯氧基自由基随后进行偶联和聚合反应,生成二聚和低聚中间体,这些中间体最终积聚在生物炭表面并被去除。这种独特的非矿化氧化实现了超高的电子利用效率(e phenols / e PDS) 的 182%。通过生物炭分子建模和理论计算,我们强调了石墨域而非氧化还原活性部分在降低带隙能量以促进电子转移方面的关键作用。我们的工作提供了对与非自由基氧化相关的突出矛盾和争议的见解,并为更多节省氧化剂的修复技术提供了灵感。


























 京公网安备 11010802027423号
京公网安备 11010802027423号