Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Weak-Coordination Electrolyte Enabling Fast Li+ Transport in Lithium Metal Batteries at Ultra-Low Temperature
Small ( IF 13.3 ) Pub Date : 2023-03-08 , DOI: 10.1002/smll.202207093 Wang Lin 1 , Jidao Li 2 , Jingshu Wang 2 , Kecheng Gu 1 , Heng Li 3 , Zhu Xu 4 , Kexuan Wang 4 , Feng Wang 4 , Mengyu Zhu 2 , You Fan 2 , Huibo Wang 4 , Guangjian Tao 1 , Na Liu 1 , Maofeng Ding 1 , Shi Chen 4 , Jiang Wu 1 , Yuxin Tang 2, 5
Small ( IF 13.3 ) Pub Date : 2023-03-08 , DOI: 10.1002/smll.202207093 Wang Lin 1 , Jidao Li 2 , Jingshu Wang 2 , Kecheng Gu 1 , Heng Li 3 , Zhu Xu 4 , Kexuan Wang 4 , Feng Wang 4 , Mengyu Zhu 2 , You Fan 2 , Huibo Wang 4 , Guangjian Tao 1 , Na Liu 1 , Maofeng Ding 1 , Shi Chen 4 , Jiang Wu 1 , Yuxin Tang 2, 5
Affiliation
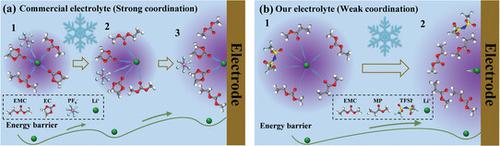
|
Lithium metal batteries (LMBs) are promising for next-generation high-energy-density batteries owing to the highest specific capacity and the lowest potential of Li metal anode. However, the LMBs are normally confronted with drastic capacity fading under extremely cold conditions mainly due to the freezing issue and sluggish Li+ desolvation process in commercial ethylene carbonate (EC)-based electrolyte at ultra-low temperature (e.g., below −30 °C). To overcome the above challenges, an anti-freezing carboxylic ester of methyl propionate (MP)-based electrolyte with weak Li+ coordination and low-freezing temperature (below −60 °C) is designed, and the corresponding LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode exhibits a higher discharge capacity of 84.2 mAh g−1 and energy density of 195.0 Wh kg−1cathode than that of the cathode (1.6 mAh g−1 and 3.9 Wh kg−1cathode) working in commercial EC-based electrolytes for NCM811‖ Li cell at −60 °C. Molecular dynamics simulation, Raman spectra, and nuclear magnetic resonance characterizations reveal that rich mobile Li+ and the unique solvation structure with weak Li+ coordination are achieved in MP-based electrolyte, which collectively facilitate the Li+ transference process at low temperature. This work provides fundamental insights into low-temperature electrolytes by regulating solvation structure, and offers the basic guidelines for the design of low-temperature electrolytes for LMBs.
中文翻译:

弱配位电解质可在超低温下实现锂金属电池中的快速 Li+ 传输
由于锂金属负极具有最高的比容量和最低的电位,锂金属电池(LMB)有望成为下一代高能量密度电池。然而,LMBs 通常在极冷条件下面临急剧的容量衰减,这主要是由于商业碳酸亚乙酯 (EC) 基电解质在超低温(例如低于 -30 °C)下的冷冻问题和缓慢的 Li +去溶剂化过程). 为克服上述挑战,设计了一种弱Li +配位、低凝固温度(-60℃以下)的丙酸甲酯(MP)基电解质的抗冻羧酸酯,对应的LiNi 0.8 Co 0.1 Mn 0.1氧2(NCM811 )正极的放电容量为 84.2 mAh g -1 ,能量密度为 195.0 Wh kg -1 NCM811‖ 锂电池在-60 °C 时的电解质。分子动力学模拟、拉曼光谱和核磁共振表征表明,在基于 MP 的电解质中实现了丰富的移动 Li +和具有弱 Li +配位的独特溶剂化结构,共同促进了 Li +低温转移过程。这项工作通过调节溶剂化结构提供了对低温电解质的基本见解,并为 LMB 的低温电解质设计提供了基本指导。
更新日期:2023-03-08
中文翻译:

弱配位电解质可在超低温下实现锂金属电池中的快速 Li+ 传输
由于锂金属负极具有最高的比容量和最低的电位,锂金属电池(LMB)有望成为下一代高能量密度电池。然而,LMBs 通常在极冷条件下面临急剧的容量衰减,这主要是由于商业碳酸亚乙酯 (EC) 基电解质在超低温(例如低于 -30 °C)下的冷冻问题和缓慢的 Li +去溶剂化过程). 为克服上述挑战,设计了一种弱Li +配位、低凝固温度(-60℃以下)的丙酸甲酯(MP)基电解质的抗冻羧酸酯,对应的LiNi 0.8 Co 0.1 Mn 0.1氧2(NCM811 )正极的放电容量为 84.2 mAh g -1 ,能量密度为 195.0 Wh kg -1 NCM811‖ 锂电池在-60 °C 时的电解质。分子动力学模拟、拉曼光谱和核磁共振表征表明,在基于 MP 的电解质中实现了丰富的移动 Li +和具有弱 Li +配位的独特溶剂化结构,共同促进了 Li +低温转移过程。这项工作通过调节溶剂化结构提供了对低温电解质的基本见解,并为 LMB 的低温电解质设计提供了基本指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号