Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2023-03-07 , DOI: 10.1016/j.aca.2023.341043 Qingqing Zou 1 , Bin Du 1 , Qianqian Zhang 1 , Hongqiang Wang 1 , Mingwan Zhang 1 , Xiaohai Yang 1 , Qing Wang 1 , Kemin Wang 1
|
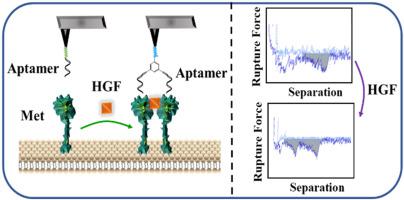
Monitoring the dimerization state of the mesenchymal-epithelial transition factor (Met) was essential for in-depth understanding of the tumor signal transduction network. At present, the dimerization activation pathway of Met protein was mainly studied at the macro level, while the research at the single molecule level was far from comprehensive. Herein, the dimerization activation of Met protein's extracellular domain induced by ligand hepatocyte growth factor (HGF) was dynamically studied by single-molecule force spectroscopy. Met protein was immobilized on a biomimetic lipid membrane for ensuring its physiological environment, and then the Met dimers were recognized by bivalent probe which was formed by two Met-binding aptamers. Then the dimeric state of Met protein could be distinguished from monomeric state of Met protein through some parameters, (such as unimodal ratio, bimodal ratio and separation work). The unimodal indicates the occurrence of single molecule binding event, and the bimodal represents the occurrence of double binding event (also represents the presence of Met dimer). Before HGF treatment, most of the Met protein on the lipid membrane was still in the form of monomer, so the unimodal ratio in the force curve was larger (78.8 ± 5.2%), and the bimodal ratio was smaller (17.0 ± 4.1%). After HGF treatment, the unimodal ratio decreased to 54.0 ± 7.4%, and the bimodal ratio increased to 43.2 ± 7.3%. It was due to the formation of dimers after the binding of Met protein on the fluidity lipid membrane with HGF. In addition, the average separation work increased to about 2 times after HGF treatment. Given that studies of Met protein dimerization inhibitors have contributed to the development of more potent and safe inhibitors to significantly inhibit tumor metastasis, the effects of different medicines (including anticoagulant medicines, different antibiotics and anti-cancer medicines) on the dimerization activation of Met protein were then explored by the platform described above. The results showed that anticoagulant medicines heparin and its analogs can significantly inhibit HGF-mediated Met protein activation, while different antibiotics and anticancer medicines had no significant effect on the dimerization of Met protein. This work provided a platform for studying protein dimerization as well as for screening Met protein dimerization inhibitors at the single-molecule level.
中文翻译:

单分子力谱研究蛋白质二聚化及药效评价
监测间充质上皮转化因子 (Met) 的二聚化状态对于深入了解肿瘤信号转导网络至关重要。目前对Met蛋白二聚化激活途径的研究主要集中在宏观层面,而单分子水平的研究还很不全面。在此,通过单分子力谱动态研究了配体肝细胞生长因子 (HGF) 诱导的 Met 蛋白胞外结构域的二聚化激活。将Met蛋白固定在仿生脂质膜上以确保其生理环境,然后Met二聚体被两个Met结合适配体形成的二价探针识别。然后可以通过一些参数(如单峰比、双峰比和分离功)将 Met 蛋白的二聚态与单体态区分开来。单峰表示单分子结合事件的发生,双峰表示双结合事件的发生(也代表Met二聚体的存在)。HGF处理前,脂膜上的Met蛋白大部分仍以单体形式存在,因此力曲线中单峰比值较大(78.8±5.2%),双峰比值较小(17.0±4.1%) . HGF 治疗后,单峰比下降至 54.0 ± 7.4%,双峰比增加至 43.2 ± 7.3%。这是由于流动性脂膜上的Met蛋白与HGF结合后形成二聚体所致。此外,HGF处理后平均分离功增加到2倍左右。鉴于对 Met 蛋白二聚化抑制剂的研究有助于开发更有效和更安全的抑制剂以显着抑制肿瘤转移,不同药物(包括抗凝药物、不同抗生素和抗癌药物)对 Met 蛋白二聚化激活的影响然后通过上述平台进行探索。结果表明,抗凝药物肝素及其类似物可显着抑制HGF介导的Met蛋白活化,而不同抗生素和抗癌药物对Met蛋白二聚化无显着影响。

























 京公网安备 11010802027423号
京公网安备 11010802027423号