Structure ( IF 5.7 ) Pub Date : 2023-03-06 , DOI: 10.1016/j.str.2023.02.008 Kishore Babu Bobbili 1 , Nukathoti Sivaji 1 , Badma Priya 1 , Kaza Suguna 1 , Avadhesha Surolia 1
|
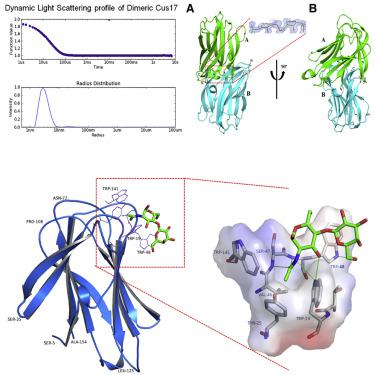
Phloem protein 2 (PP2) contributes crucially to phloem-based defense in plants by binding to carbohydrates displayed by pathogens. However, its three-dimensional structure and the sugar binding site remained unexplored. Here, we report the crystal structure of the dimeric PP2 Cus17 from Cucumis sativus in its apo form and complexed with nitrobenzene, N-acetyllactosamine, and chitotriose. Each protomer of Cus17 consists of two antiparallel four-stranded twisted β sheets, a β hairpin, and three short helices forming a β sandwich architectural fold. This structural fold has not been previously observed in other plant lectin families. Structure analysis of the lectin-carbohydrate complexes reveals an extended carbohydrate binding site in Cus17, composed mostly of aromatic amino acids. Our studies suggest a highly conserved tertiary structure and a versatile binding site capable of recognizing motifs common to diverse glycans on plant pathogens/pests, which makes the PP2 family suited for phloem-based plant defense.
中文翻译:

黄瓜韧皮部凝集素(韧皮部蛋白 2)Cus17 的结构和相互作用
韧皮部蛋白 2 (PP2) 通过与病原体展示的碳水化合物结合,对植物中基于韧皮部的防御至关重要。然而,其三维结构和糖结合位点仍未得到探索。在这里,我们报告了来自黄瓜的二聚体 PP2 Cus17 的晶体结构以其载脂蛋白形式与硝基苯、N-乙酰乳糖胺和壳三糖络合。Cus17 的每个原聚体由两个反平行的四链扭曲 β 折叠、一个 β 发夹和三个形成 β 三明治结构折叠的短螺旋组成。这种结构折叠以前没有在其他植物凝集素家族中观察到。凝集素-碳水化合物复合物的结构分析揭示了 Cus17 中一个扩展的碳水化合物结合位点,主要由芳香族氨基酸组成。我们的研究表明高度保守的三级结构和多功能结合位点能够识别植物病原体/害虫上多种聚糖共有的基序,这使得 PP2 家族适合基于韧皮部的植物防御。


























 京公网安备 11010802027423号
京公网安备 11010802027423号