Structure ( IF 5.7 ) Pub Date : 2023-03-03 , DOI: 10.1016/j.str.2023.02.003 Tania M Palhano Zanela 1 , Alexzandrea Woudenberg 1 , Karen G Romero Bello 1 , Eric S Underbakke 1
|
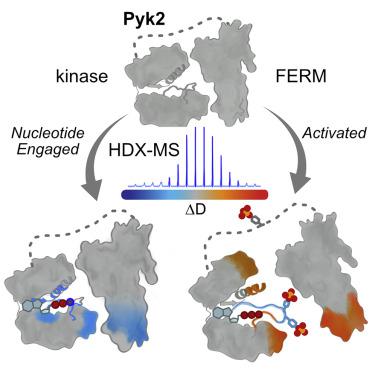
Pyk2 is a multidomain non-receptor tyrosine kinase that undergoes a multistage activation mechanism. Activation is instigated by conformational rearrangements relieving autoinhibitory FERM domain interactions. The kinase autophosphorylates a central linker residue to recruit Src kinase. Pyk2 and Src mutually phosphorylate activation loops to confer full activation. While the mechanisms of autoinhibition are established, the conformational dynamics associated with autophosphorylation and Src recruitment remain unclear. We employ hydrogen/deuterium exchange mass spectrometry and kinase activity profiling to map the conformational dynamics associated with substrate binding and Src-mediated activation loop phosphorylation. Nucleotide engagement stabilizes the autoinhibitory interface, while phosphorylation deprotects both FERM and kinase regulatory surfaces. Phosphorylation organizes active site motifs linking catalytic loop with activation segment. Dynamics of the activation segment anchor propagate to EF/G helices to prevent reversion of the autoinhibitory FERM interaction. We employ targeted mutagenesis to dissect how phosphorylation-induced conformational rearrangements elevate kinase activity above the basal autophosphorylation rate.
中文翻译:

激活环磷酸化调节 Pyk2 酪氨酸激酶激活的构象动力学
Pyk2 是一种多域非受体酪氨酸激酶,经历多阶段激活机制。激活是由缓解自身抑制性 FERM 域相互作用的构象重排引起的。激酶自磷酸化中央接头残基以募集 Src 激酶。Pyk2 和 Src 相互磷酸化激活环以赋予完全激活。虽然建立了自身抑制机制,但与自身磷酸化和 Src 募集相关的构象动力学仍不清楚。我们采用氢/氘交换质谱和激酶活性分析来绘制与底物结合和 Src 介导的激活环磷酸化相关的构象动力学。核苷酸结合稳定了自抑制界面,而磷酸化使 FERM 和激酶调节表面脱保护。磷酸化组织连接催化环和激活片段的活性位点基序。激活片段锚的动力学传播到 EF/G 螺旋以防止自抑制 FERM 相互作用的逆转。我们采用靶向诱变来剖析磷酸化诱导的构象重排如何将激酶活性提高到基础自磷酸化率之上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号