当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Convergent Synthesis of HPK1 Inhibitor GNE-6893 via Palladium-Catalyzed Functionalization of a Tetrasubstituted Isoquinoline
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-02-27 , DOI: 10.1021/acs.oprd.2c00384 Andreas Stumpf 1 , Di Xu 1 , Rohit Ranjan 1 , Remy Angelaud 1 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-02-27 , DOI: 10.1021/acs.oprd.2c00384 Andreas Stumpf 1 , Di Xu 1 , Rohit Ranjan 1 , Remy Angelaud 1 , Francis Gosselin 1
Affiliation
|
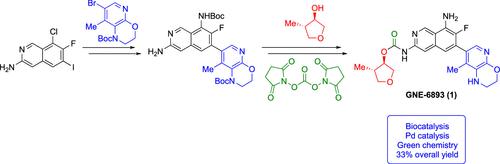
A scalable convergent synthesis of GNE-6893 (1) involving Pd-catalyzed Suzuki–Miyaura cross-coupling and C–N coupling as key steps is reported. The production of the final active pharmaceutical ingredient (API) was achieved in 33% overall yield and 98 A% HPLC purity by a one-pot process of global deprotection, pH adjustment, crystallization, and isolation. Green chemistry practices were implemented in the process development of GNE-6893 (1) by utilizing a biocatalytic resolution to produce (3R,4S)-4-methyltetrahydrofuran-3-ol and replacing toxic triphosgene with a safer alternative, disuccinimidyl carbonate (DSC), to construct the carbamate penultimate intermediate to API.
中文翻译:

HPK1 抑制剂 GNE-6893 的聚合合成通过四取代异喹啉的钯催化功能化
报道了一种可扩展的GNE-6893会聚合成( 1 ),其中 Pd 催化的 Suzuki–Miyaura 交叉偶联和 C–N 偶联作为关键步骤。通过全局脱保护、pH 调节、结晶和分离的一锅法工艺,实现了最终活性药物成分 (API) 的生产,总收率为 33%,HPLC 纯度为 98 A%。在GNE-6893 ( 1 )的工艺开发过程中实施了绿色化学实践,利用生物催化拆分生产 (3 R ,4 S )-4-甲基四氢呋喃-3-醇,并用更安全的替代品碳酸二琥珀酰亚胺酯替代有毒的三光气 ( DSC), 以构建 API 的氨基甲酸酯倒数第二个中间体。
更新日期:2023-02-27
中文翻译:

HPK1 抑制剂 GNE-6893 的聚合合成通过四取代异喹啉的钯催化功能化
报道了一种可扩展的GNE-6893会聚合成( 1 ),其中 Pd 催化的 Suzuki–Miyaura 交叉偶联和 C–N 偶联作为关键步骤。通过全局脱保护、pH 调节、结晶和分离的一锅法工艺,实现了最终活性药物成分 (API) 的生产,总收率为 33%,HPLC 纯度为 98 A%。在GNE-6893 ( 1 )的工艺开发过程中实施了绿色化学实践,利用生物催化拆分生产 (3 R ,4 S )-4-甲基四氢呋喃-3-醇,并用更安全的替代品碳酸二琥珀酰亚胺酯替代有毒的三光气 ( DSC), 以构建 API 的氨基甲酸酯倒数第二个中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号