当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous Characterization of Reaction Kinetics and Enthalpy by Calorimetry Based on Spatially Resolved Temperature Profile in Flow Reactors
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-02-10 , DOI: 10.1021/acs.oprd.2c00251 Yusuke Imamura 1 , Jun-ichi Ogawa 1 , Yuma Otake 1 , Hidenosuke Itoh 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-02-10 , DOI: 10.1021/acs.oprd.2c00251 Yusuke Imamura 1 , Jun-ichi Ogawa 1 , Yuma Otake 1 , Hidenosuke Itoh 1
Affiliation
|
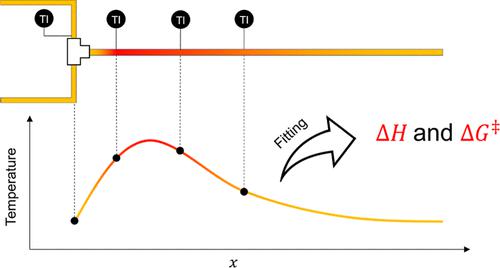
Characterizing the Gibbs energy of activation and the enthalpy of reactions typically requires repeated sampling. In this study, a new calorimetric reaction-characterization technique was developed for thermokinetic analysis in a flow reactor. The method was used to simultaneously determine the Gibbs energy of activation from the reaction kinetics and the enthalpy of reaction by fitting the thermal model equation to the spatially resolved temperature profile obtained on a flow reactor. This procedure was verified and comparatively assessed with conventional analytical methods using a family of peptide syntheses as model reactions. The thermal model equation closely fitted the acquired temperature profile. The calculated Gibbs energies of activation agreed closely with the values determined from product yield-time trends obtained using several reactants at different temperatures. The calculated reaction enthalpy was similar to the values obtained from quantum chemical calculations. The findings suggest that spatial regression calorimetry can simultaneously extract more reaction characteristics than conventional calorimetry.
中文翻译:

基于流动反应器中空间分辨温度分布的量热法同时表征反应动力学和热焓
表征吉布斯活化能和反应焓通常需要重复采样。在这项研究中,开发了一种新的量热反应表征技术,用于流动反应器中的热动力学分析。该方法用于通过将热模型方程拟合到在流动反应器上获得的空间分辨温度曲线,同时从反应动力学和反应焓中确定吉布斯活化能。使用一系列肽合成作为模型反应,使用常规分析方法对该程序进行了验证和比较评估。热模型方程与获取的温度曲线非常吻合。计算出的吉布斯活化能与根据在不同温度下使用多种反应物获得的产品屈服时间趋势确定的值非常吻合。计算的反应焓类似于从量子化学计算获得的值。研究结果表明,空间回归量热法可以同时提取比传统量热法更多的反应特征。
更新日期:2023-02-10
中文翻译:

基于流动反应器中空间分辨温度分布的量热法同时表征反应动力学和热焓
表征吉布斯活化能和反应焓通常需要重复采样。在这项研究中,开发了一种新的量热反应表征技术,用于流动反应器中的热动力学分析。该方法用于通过将热模型方程拟合到在流动反应器上获得的空间分辨温度曲线,同时从反应动力学和反应焓中确定吉布斯活化能。使用一系列肽合成作为模型反应,使用常规分析方法对该程序进行了验证和比较评估。热模型方程与获取的温度曲线非常吻合。计算出的吉布斯活化能与根据在不同温度下使用多种反应物获得的产品屈服时间趋势确定的值非常吻合。计算的反应焓类似于从量子化学计算获得的值。研究结果表明,空间回归量热法可以同时提取比传统量热法更多的反应特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号