Cell ( IF 64.5 ) Pub Date : 2023-02-09 , DOI: 10.1016/j.cell.2023.01.012 Brianna Duncan-Lowey 1 , Nitzan Tal 2 , Alex G Johnson 1 , Shaun Rawson 3 , Megan L Mayer 3 , Shany Doron 2 , Adi Millman 2 , Sarah Melamed 2 , Taya Fedorenko 2 , Assaf Kacen 4 , Alexander Brandis 5 , Tevie Mehlman 5 , Gil Amitai 2 , Rotem Sorek 2 , Philip J Kranzusch 6
|
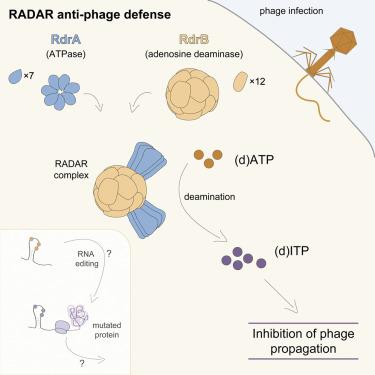
RADAR is a two-protein bacterial defense system that was reported to defend against phage by “editing” messenger RNA. Here, we determine cryo-EM structures of the RADAR defense complex, revealing RdrA as a heptameric, two-layered AAA+ ATPase and RdrB as a dodecameric, hollow complex with twelve surface-exposed deaminase active sites. RdrA and RdrB join to form a giant assembly up to 10 MDa, with RdrA docked as a funnel over the RdrB active site. Surprisingly, our structures reveal an RdrB active site that targets mononucleotides. We show that RdrB catalyzes ATP-to-ITP conversion in vitro and induces the massive accumulation of inosine mononucleotides during phage infection in vivo, limiting phage replication. Our results define ATP mononucleotide deamination as a determinant of RADAR immunity and reveal supramolecular assembly of a nucleotide-modifying machine as a mechanism of anti-phage defense.
中文翻译:

RADAR超分子抗噬菌体防御复合物的冷冻电镜结构
RADAR 是一种双蛋白细菌防御系统,据报道可通过“编辑”信使 RNA 来防御噬菌体。在这里,我们确定了 RADAR 防御复合体的低温 EM 结构,揭示了 RdrA 是七聚体、双层 AAA+ ATP 酶,而 RdrB 是十二聚体、空心复合体,具有十二个表面暴露的脱氨酶活性位点。RdrA 和 RdrB 结合形成一个高达 10 MDa 的巨型组件,RdrA 作为漏斗停靠在 RdrB 活性位点上。令人惊讶的是,我们的结构揭示了一个靶向单核苷酸的 RdrB 活性位点。我们表明 RdrB在体外催化 ATP 到 ITP 的转化,并在体内噬菌体感染期间诱导肌苷单核苷酸的大量积累, 限制噬菌体复制。我们的结果将 ATP 单核苷酸脱氨基定义为雷达免疫的决定因素,并揭示了核苷酸修饰机器的超分子组装是一种抗噬菌体防御机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号