当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantio- and Diastereoselective De Novo Synthesis of 3-Substituted Proline Derivatives via Cooperative Photoredox/Brønsted Acid Catalysis and Epimerization
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-01-27 , DOI: 10.1021/jacs.2c12995 Chao Che 1 , Yi-Nan Lu 1 , Chun-Jiang Wang 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-01-27 , DOI: 10.1021/jacs.2c12995 Chao Che 1 , Yi-Nan Lu 1 , Chun-Jiang Wang 1, 2
Affiliation
|
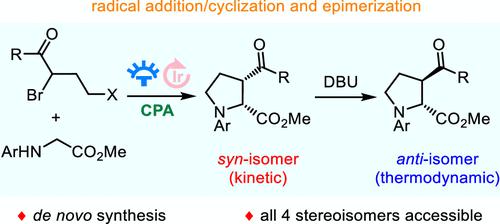
Herein, a novel strategy for the catalytic asymmetric synthesis of enantioenriched 3-cis- and 3-trans-substituted prolines has been successfully established via an unprecedented cascade radical addition/cyclization enabled by synergistic photoredox/Brønsted acid catalysis and subsequent base-assisted epimerization. The current protocol provides a unique de novo access to all four stereoisomers of 3-substituted prolines which are not readily achieved via currently established methods. This methodology could be further extended to the asymmetric synthesis of the full complement of stereoisomers of 3-substituted pipecolinic acids.
中文翻译:

3-取代脯氨酸衍生物的对映和非对映选择性从头合成通过协同光氧化还原/布朗斯特酸催化和差向异构化
在此,通过协同光氧化还原/布朗斯台德酸催化和随后的碱辅助差向异构化实现的前所未有的级联自由基加成/环化,成功建立了催化不对称合成富含对映体的 3-顺式和 3-反式脯氨酸的新策略。目前的协议提供了对 3-取代脯氨酸的所有四种立体异构体的独特从头访问,这些异构体通过目前建立的方法不容易实现。该方法可以进一步扩展到 3-取代哌啶酸的完整立体异构体的不对称合成。
更新日期:2023-01-27
中文翻译:

3-取代脯氨酸衍生物的对映和非对映选择性从头合成通过协同光氧化还原/布朗斯特酸催化和差向异构化
在此,通过协同光氧化还原/布朗斯台德酸催化和随后的碱辅助差向异构化实现的前所未有的级联自由基加成/环化,成功建立了催化不对称合成富含对映体的 3-顺式和 3-反式脯氨酸的新策略。目前的协议提供了对 3-取代脯氨酸的所有四种立体异构体的独特从头访问,这些异构体通过目前建立的方法不容易实现。该方法可以进一步扩展到 3-取代哌啶酸的完整立体异构体的不对称合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号