当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Transformation of Colloidal Cs3Cu2Cl5 Nanocrystals to CsMCl (M = Zn, Bi, Cd) by Cation Exchange and Their Thermodynamic Study by Density Functional Theory Calculations
Chemistry of Materials ( IF 8.6 ) Pub Date : 2023-01-25 , DOI: 10.1021/acs.chemmater.2c03336 Hyo-Geun Kwon, Seung Min Lee, Jehyeon Ryu, Ju Hyun Park, Sang Kyu Kwak, Sang-Wook Kim
Chemistry of Materials ( IF 8.6 ) Pub Date : 2023-01-25 , DOI: 10.1021/acs.chemmater.2c03336 Hyo-Geun Kwon, Seung Min Lee, Jehyeon Ryu, Ju Hyun Park, Sang Kyu Kwak, Sang-Wook Kim
|
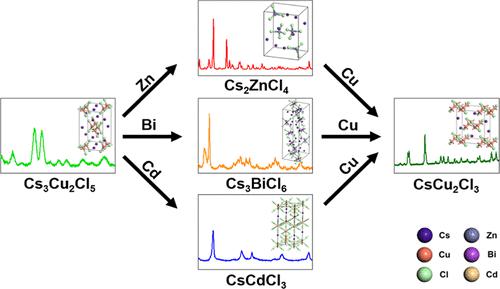
We present the phase transformation from colloidal Cs3Cu2Cl5 nanocrystals to CsMCl (M = Zn, Bi, Cd) by cation exchange reaction. Cs2ZnCl4, Cs3BiCl6, and CsCdCl3 were successfully synthesized, and the feasibility of phase transformations was demonstrated using density functional theory calculations, which revealed the high thermodynamic stability of the three structures. The results indicate that these structures can be synthetically prepared. The difference in reactivity between Zn, Bi, and Cd cations, which was verified by changing the reaction temperatures, was demonstrated using chemical softness calculations considering the interactions between Cl– and three cations. Additionally, for each cation exchange reaction, thermodynamic stability, estimated in terms of the formation energy, contributed to reactivity. The Cs2ZnCl4 structure required the mildest reaction condition (i.e., 110 °C). As a reverse reaction, Cu cations were added to solutions of Cs2ZnCl4, Cs3BiCl6, and CsCdCl3, and CsCu2Cl3 was obtained instead of Cs3Cu2Cl5. The mechanism was not cation exchange, and transmission electron microscopy data showed that nanoparticles were used as precursors for forming CsCu2Cl3 particles.
中文翻译:

通过阳离子交换将胶体 Cs3Cu2Cl5 纳米晶相变为 CsMCl(M = Zn、Bi、Cd)及其通过密度泛函理论计算的热力学研究
我们介绍了通过阳离子交换反应从胶体 Cs 3 Cu 2 Cl 5纳米晶体到 CsMCl(M = Zn、Bi、Cd)的相变。Cs 2 ZnCl 4、Cs 3 BiCl 6和CsCdCl 3成功合成,并利用密度泛函理论计算证明了相变的可行性,揭示了三种结构的高热力学稳定性。结果表明这些结构可以合成制备。通过改变反应温度验证了 Zn、Bi 和 Cd 阳离子之间反应性的差异,并通过考虑 Cl -和三种阳离子之间相互作用的化学软度计算证明了这一点。此外,对于每个阳离子交换反应,根据形成能估计的热力学稳定性有助于反应性。Cs 2 ZnCl 4结构需要最温和的反应条件(即 110 °C)。作为逆反应,将Cu阳离子加入到Cs 2 ZnCl 4、Cs 3 BiCl 6和CsCdCl 3的溶液中,得到CsCu 2 Cl 3而不是Cs 3 Cu 2 Cl 5。该机制不是阳离子交换,并且透射电子显微镜数据显示纳米颗粒被用作形成CsCu 2 Cl 3颗粒的前体。
更新日期:2023-01-25
中文翻译:

通过阳离子交换将胶体 Cs3Cu2Cl5 纳米晶相变为 CsMCl(M = Zn、Bi、Cd)及其通过密度泛函理论计算的热力学研究
我们介绍了通过阳离子交换反应从胶体 Cs 3 Cu 2 Cl 5纳米晶体到 CsMCl(M = Zn、Bi、Cd)的相变。Cs 2 ZnCl 4、Cs 3 BiCl 6和CsCdCl 3成功合成,并利用密度泛函理论计算证明了相变的可行性,揭示了三种结构的高热力学稳定性。结果表明这些结构可以合成制备。通过改变反应温度验证了 Zn、Bi 和 Cd 阳离子之间反应性的差异,并通过考虑 Cl -和三种阳离子之间相互作用的化学软度计算证明了这一点。此外,对于每个阳离子交换反应,根据形成能估计的热力学稳定性有助于反应性。Cs 2 ZnCl 4结构需要最温和的反应条件(即 110 °C)。作为逆反应,将Cu阳离子加入到Cs 2 ZnCl 4、Cs 3 BiCl 6和CsCdCl 3的溶液中,得到CsCu 2 Cl 3而不是Cs 3 Cu 2 Cl 5。该机制不是阳离子交换,并且透射电子显微镜数据显示纳米颗粒被用作形成CsCu 2 Cl 3颗粒的前体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号