当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chalcones: Potential Chemotherapeutic Compounds and Educational Tools for Closing the Loop in STEM
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2023-01-25 , DOI: 10.1021/acs.accounts.2c00583 Albert E Russell 1 , Brandon R Gines 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2023-01-25 , DOI: 10.1021/acs.accounts.2c00583 Albert E Russell 1 , Brandon R Gines 1
Affiliation
|
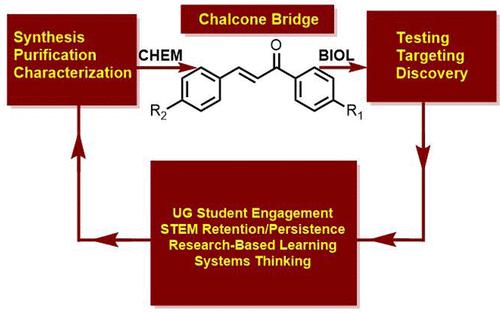
The study discussed herein describes the synthesis of halogenated chalcones as potential chemotherapeutics. The synthesis work was conducted by undergraduate students participating in an Organic Chemistry II laboratory course at Tuskegee University, while the biological assays were conducted by students enrolled in a Molecular Biology I laboratory course. Chalcones were synthesized via aldol condensation and purified from hot ethanol. The impetus for the work was the fact that Tuskegee University sits positioned within the Black Belt of Alabama which, in addition to being an area of fertile soil and excellent farmland, is also an area rife with health disparities that particularly affect African-Americans. Breast cancer, specifically triple-negative breast cancer, affects African-American women at a higher rate than any other ethnic group. The work described herein addresses a practical problem [teaching undergraduate students about the interface of synthetic techniques, synthesis of specific classes of compounds, functional groups, and their relation to biological activity], as well an existential problem [the prevalence of breast cancer among African-American women, and the need to develop targeted treatments]. One of the chief aims of this approach of integrating these ideas into our laboratory courses was to facilitate the understanding of translational science, i.e. taking chalcones from benchtop to potential therapies for breast cancer. Another aim of the current approach was to, in essence, create a research problem based course and concomitantly use the results of the experiments performed in the course as a way to address the dearth of research funding that HBCUs typically receive. The pharmacological activities of chalcones and their derivatives are well documented. They are an important class of natural products that occur in edible plant derivatives such as spices, teas, fruits and various vegetables. In vitro studies have shown that chalcones inhibit proliferation of breast cancer cells by inducing apoptosis and blocking cell progression. The synthesis of chalcones with aromatic substituents has been investigated, and electron rich chalcones, i.e., chalcones with donors attached to the aromatic rings, have been studied extensively. The effect that adding electron withdrawing groups to the chalcone structural motif has on the antiproliferation ability of chalcones had been only minimally investigated at the time that our studies were being conducted. We examined the introduction of chlorine to the aromatic system of the chalcone and how these electron withdrawing substituents affect the chalcone’s antiproliferative ability. It was discovered that (E)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one inhibited MDA-MB-231 cell progression in a dose dependent manner and outperformed the unsubstituted (E)-1,3-diphenyl-2-propen-1-one (1) at concentrations ranging from 0 μg/mL to 20 μg/mL. Cell death was determined by MTT assay.
中文翻译:

查耳酮:潜在的化疗化合物和用于关闭 STEM 循环的教育工具
本文讨论的研究描述了卤代查耳酮作为潜在化疗药物的合成。合成工作由参加塔斯基吉大学有机化学 II 实验室课程的本科生进行,而生物测定则由参加分子生物学 I 实验室课程的学生进行。查尔酮通过羟醛缩合合成并从热乙醇中纯化。这项工作的推动力是,塔斯基吉大学位于阿拉巴马州的黑带内,除了拥有肥沃的土壤和优良的农田外,还是一个充斥着特别影响非裔美国人的健康差异的地区。乳腺癌,特别是三阴性乳腺癌,对非裔美国女性的影响高于任何其他种族群体。本文描述的工作解决了一个实际问题 [教授本科生关于合成技术的界面、特定类别化合物的合成、官能团及其与生物活性的关系],以及一个存在的问题 [非洲乳腺癌的患病率] -美国女性,以及开发针对性治疗的必要性]。这种将这些想法整合到我们的实验室课程中的方法的主要目标之一是促进转化科学的理解,即将查耳酮从实验室用于乳腺癌的潜在疗法。当前方法的另一个目标是,从本质上讲,创建一个基于研究问题的课程,并同时使用课程中进行的实验结果作为解决 HBCU 通常收到的研究资金匮乏的一种方式。查耳酮及其衍生物的药理活性已得到充分证明。它们是一类重要的天然产物,存在于可食用的植物衍生物中,例如香料、茶叶、水果和各种蔬菜。体外研究表明,查尔酮通过诱导细胞凋亡和阻断细胞进展来抑制乳腺癌细胞的增殖。已经研究了具有芳族取代基的查尔酮的合成,并且已经广泛研究了富电子查尔酮,即具有连接到芳环上的供体的查尔酮。在我们进行研究时,对查耳酮结构基序添加吸电子基团对查耳酮抗增殖能力的影响仅进行了最低限度的研究。我们研究了将氯引入查尔酮的芳香系统,以及这些吸电子取代基如何影响查尔酮的抗增殖能力。结果发现 ( E)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one 以剂量依赖性方式抑制 MDA-MB-231 细胞进展,优于未取代的 (E)-1,3-diphenyl - 2- propen-1-one (1) 的浓度范围为 0 μg/mL 至 20 μg/mL。通过MTT测定法确定细胞死亡。
更新日期:2023-01-25
中文翻译:

查耳酮:潜在的化疗化合物和用于关闭 STEM 循环的教育工具
本文讨论的研究描述了卤代查耳酮作为潜在化疗药物的合成。合成工作由参加塔斯基吉大学有机化学 II 实验室课程的本科生进行,而生物测定则由参加分子生物学 I 实验室课程的学生进行。查尔酮通过羟醛缩合合成并从热乙醇中纯化。这项工作的推动力是,塔斯基吉大学位于阿拉巴马州的黑带内,除了拥有肥沃的土壤和优良的农田外,还是一个充斥着特别影响非裔美国人的健康差异的地区。乳腺癌,特别是三阴性乳腺癌,对非裔美国女性的影响高于任何其他种族群体。本文描述的工作解决了一个实际问题 [教授本科生关于合成技术的界面、特定类别化合物的合成、官能团及其与生物活性的关系],以及一个存在的问题 [非洲乳腺癌的患病率] -美国女性,以及开发针对性治疗的必要性]。这种将这些想法整合到我们的实验室课程中的方法的主要目标之一是促进转化科学的理解,即将查耳酮从实验室用于乳腺癌的潜在疗法。当前方法的另一个目标是,从本质上讲,创建一个基于研究问题的课程,并同时使用课程中进行的实验结果作为解决 HBCU 通常收到的研究资金匮乏的一种方式。查耳酮及其衍生物的药理活性已得到充分证明。它们是一类重要的天然产物,存在于可食用的植物衍生物中,例如香料、茶叶、水果和各种蔬菜。体外研究表明,查尔酮通过诱导细胞凋亡和阻断细胞进展来抑制乳腺癌细胞的增殖。已经研究了具有芳族取代基的查尔酮的合成,并且已经广泛研究了富电子查尔酮,即具有连接到芳环上的供体的查尔酮。在我们进行研究时,对查耳酮结构基序添加吸电子基团对查耳酮抗增殖能力的影响仅进行了最低限度的研究。我们研究了将氯引入查尔酮的芳香系统,以及这些吸电子取代基如何影响查尔酮的抗增殖能力。结果发现 ( E)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one 以剂量依赖性方式抑制 MDA-MB-231 细胞进展,优于未取代的 (E)-1,3-diphenyl - 2- propen-1-one (1) 的浓度范围为 0 μg/mL 至 20 μg/mL。通过MTT测定法确定细胞死亡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号