当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Generation of 1,2-Difluorobenzene via a Photochemical Fluorodediazoniation Step in a Continuous Flow Mode
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-16 , DOI: 10.1021/acs.oprd.2c00348 Kevin Simon 1, 2 , Desiree Znidar 1, 2 , Julien Boutet 3 , Gérard Guillamot 3 , Jean-Yves Lenoir 3 , Doris Dallinger 1, 2 , C. Oliver Kappe 1, 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-16 , DOI: 10.1021/acs.oprd.2c00348 Kevin Simon 1, 2 , Desiree Znidar 1, 2 , Julien Boutet 3 , Gérard Guillamot 3 , Jean-Yves Lenoir 3 , Doris Dallinger 1, 2 , C. Oliver Kappe 1, 2
Affiliation
|
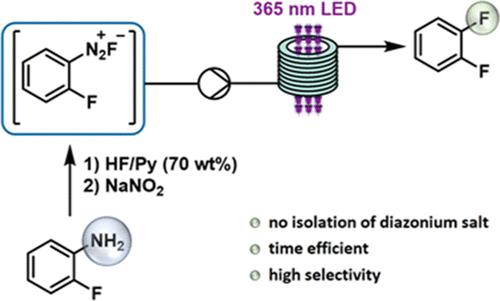
A proof-of-concept study for the synthesis of 1,2-difluorobenzene from 2-fluoroaniline via the Balz–Schiemann reaction using HF/pyridine as the fluorinating reagent is reported. Key to success for a fast reaction, a clean reaction profile─and thus high product selectivity─was a photochemically induced fluorodediazoniation of the in situ-generated diazonium salt performed in a continuous flow mode. A high-power 365 nm light-emitting diode provided a more robust and efficient irradiation system compared to a medium-pressure Hg lamp with respect to the reaction performance on scale-out runs and reaction time, allowing the generation of 1,2-difluorobenzene within a 10 min residence time and a product selectivity of ≥95% at full conversion.
中文翻译:

在连续流动模式下通过光化学氟化重氮化步骤生成 1,2-二氟苯
报道了使用 HF/吡啶作为氟化试剂,通过 Balz-Schiemann 反应从 2-氟苯胺合成 1,2-二氟苯的概念验证研究。在连续流动模式下对原位生成的重氮盐进行光化学诱导的氟脱重氮反应是实现快速反应、干净的反应曲线以及高产品选择性的成功关键。与中压汞灯相比,高功率 365 nm 发光二极管在横向扩展运行的反应性能和反应时间方面提供了更强大、更高效的照射系统,从而可以生成 1,2-二氟苯在 10 分钟的停留时间内,在完全转化时产品选择性≥95%。
更新日期:2023-01-16
中文翻译:

在连续流动模式下通过光化学氟化重氮化步骤生成 1,2-二氟苯
报道了使用 HF/吡啶作为氟化试剂,通过 Balz-Schiemann 反应从 2-氟苯胺合成 1,2-二氟苯的概念验证研究。在连续流动模式下对原位生成的重氮盐进行光化学诱导的氟脱重氮反应是实现快速反应、干净的反应曲线以及高产品选择性的成功关键。与中压汞灯相比,高功率 365 nm 发光二极管在横向扩展运行的反应性能和反应时间方面提供了更强大、更高效的照射系统,从而可以生成 1,2-二氟苯在 10 分钟的停留时间内,在完全转化时产品选择性≥95%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号