当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transformation of the Manufacturing Process from Discovery to Kilogram Scale for AWZ1066S: A Highly Specific Anti-Wolbachia Drug Candidate for a Short-Course Treatment of Filariasis
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-10 , DOI: 10.1021/acs.oprd.2c00167 W. David Hong 1 , Paul M. O’Neill 1 , Mark J. Taylor 2 , Joseph D. Turner 2 , Stephen A. Ward 2 , Fabian Gusovsky 3 , Farid Benayoud 4 , Nao Shibuguchi 5 , Dixit Girish 6 , Francis Gerard Fang 4 , Ravi Kumar Talabhakthla 6 , Anand Vaddi 6 , Chiranjeevi Challa 6 , Karri Satya Ammi Reddy 6 , Vijay Kalla 6 , Sugandham Srinivasa Rao 6 , Durga Mahesh Kumar Nagireddi 6 , Jayesh Patel 6 , Anil Shahaji Khile 6
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-10 , DOI: 10.1021/acs.oprd.2c00167 W. David Hong 1 , Paul M. O’Neill 1 , Mark J. Taylor 2 , Joseph D. Turner 2 , Stephen A. Ward 2 , Fabian Gusovsky 3 , Farid Benayoud 4 , Nao Shibuguchi 5 , Dixit Girish 6 , Francis Gerard Fang 4 , Ravi Kumar Talabhakthla 6 , Anand Vaddi 6 , Chiranjeevi Challa 6 , Karri Satya Ammi Reddy 6 , Vijay Kalla 6 , Sugandham Srinivasa Rao 6 , Durga Mahesh Kumar Nagireddi 6 , Jayesh Patel 6 , Anil Shahaji Khile 6
Affiliation
|
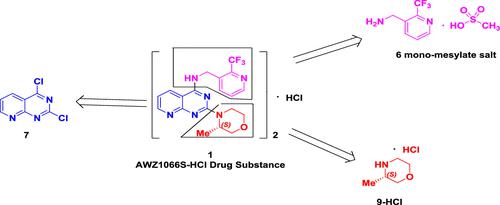
Anti-Wolbachia therapy has been clinically proven to be a safe approach for the treatment of onchocerciasis and lymphatic filariasis. AWZ1066S, a first-in-class highly specific anti-Wolbachia drug candidate developed for a short-course treatment of human filariasis, has advanced into clinical development. An improved, cost-efficient, and scalable process for the manufacture of this clinical candidate is described. Presented herein is the process development work for the active pharmaceutical ingredient (API) and its two key starting materials [2-(trifluoromethyl)-3-pyridyl]methanamine and (S)-3-methylmorpholine, starting from 2,4-dichloropyrido[2,3-d]pyrimidine, which is capable of delivering high-purity (>99%) API consistently. The optimized production route was used in the manufacture of the clinical candidate at the kilogram scale to support the ongoing clinical development.
中文翻译:

AWZ1066S 的制造过程从发现到公斤级的转变:一种用于丝虫病短期治疗的高度特异性抗沃尔巴克氏体候选药物
抗沃尔巴克氏体疗法已被临床证明是治疗盘尾丝虫病和淋巴丝虫病的安全方法。AWZ1066S是为短程治疗人类丝虫病而开发的first-in-class高特异性抗沃尔巴克氏体候选药物,已进入临床开发阶段。描述了用于制造该临床候选物的改进的、具有成本效益的和可扩展的过程。本文介绍的是活性药物成分 (API) 及其两种关键原料 [2-(三氟甲基)-3-吡啶基] 甲胺和 ( S )-3-甲基吗啉的工艺开发工作,从 2,4-二氯吡啶并开始[ 2,3- d]嘧啶,能够始终如一地提供高纯度 (>99%) API。优化的生产路线用于千克级临床候选药物的生产,以支持正在进行的临床开发。
更新日期:2023-01-10
中文翻译:

AWZ1066S 的制造过程从发现到公斤级的转变:一种用于丝虫病短期治疗的高度特异性抗沃尔巴克氏体候选药物
抗沃尔巴克氏体疗法已被临床证明是治疗盘尾丝虫病和淋巴丝虫病的安全方法。AWZ1066S是为短程治疗人类丝虫病而开发的first-in-class高特异性抗沃尔巴克氏体候选药物,已进入临床开发阶段。描述了用于制造该临床候选物的改进的、具有成本效益的和可扩展的过程。本文介绍的是活性药物成分 (API) 及其两种关键原料 [2-(三氟甲基)-3-吡啶基] 甲胺和 ( S )-3-甲基吗啉的工艺开发工作,从 2,4-二氯吡啶并开始[ 2,3- d]嘧啶,能够始终如一地提供高纯度 (>99%) API。优化的生产路线用于千克级临床候选药物的生产,以支持正在进行的临床开发。


























 京公网安备 11010802027423号
京公网安备 11010802027423号