当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impurity Purging through Systematic Process Development of a Continuous Two-Stage Crystallization
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-04 , DOI: 10.1021/acs.oprd.2c00317 Drew Scott 1 , Naomi E. B. Briggs 2 , Anna Formosa 2 , Annessa Burnett 1 , Bimbisar Desai 3 , Greg Hammersmith 2 , Kersten Rapp 2 , Gerard Capellades 4 , Allan S. Myerson 5 , Thomas D. Roper 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-01-04 , DOI: 10.1021/acs.oprd.2c00317 Drew Scott 1 , Naomi E. B. Briggs 2 , Anna Formosa 2 , Annessa Burnett 1 , Bimbisar Desai 3 , Greg Hammersmith 2 , Kersten Rapp 2 , Gerard Capellades 4 , Allan S. Myerson 5 , Thomas D. Roper 1
Affiliation
|
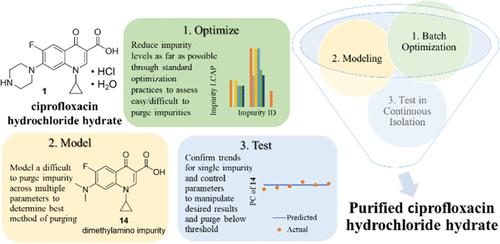
A methodical development approach was deployed in a novel portable manufacturing (Pharmacy on Demand) unit to purify ciprofloxacin hydrochloride hydrate within assay, water content, and impurity specifications described by the United States Pharmacopeia (USP) monograph and ICH Q3A(R2) guidelines for new impurities in drug substances. A series of design-of-experiment (DOE) and one-factor at a time (OFAT) experiments led to the optimization and control of a continuous two-stage crystallization that increased both the purity and yield of ciprofloxacin hydrochloride hydrate. Additionally, a statistically significant linear model was derived in batch within a 20 °C range that tracked the level of a difficult-to-purge impurity in stage 1 of the purification. This model was tested in continuous flow and predicted the impurity removal within 5% accuracy. With parametric control of process parameters, determined by optimization and modeling work, continuous flow isolations produced an active pharmaceutical ingredient (API) which had no individual impurities above 0.07%, with an isolated yield of 74%. In addition, acceptance criteria for assay (between 98 and 102%) and water content (between 4.7 and 6.7%) were met per the USP monograph for ciprofloxacin hydrochloride hydrate for the first time in the novel POD system.
中文翻译:

通过连续两级结晶的系统工艺开发清除杂质
在新型便携式制造(按需制药)装置中部署了一种有条不紊的开发方法,以在美国药典 (USP) 专论和 ICH Q3A(R2) 新指南描述的含量、水含量和杂质规格范围内纯化盐酸环丙沙星水合物原料药中的杂质。一系列实验设计 (DOE) 和一次一个因素 (OFAT) 实验导致连续两阶段结晶的优化和控制,从而提高了盐酸环丙沙星水合物的纯度和收率。此外,在 20 °C 范围内批量导出具有统计学意义的线性模型,该模型跟踪纯化阶段 1 中难以清除的杂质的水平。该模型在连续流中进行了测试,预测的杂质去除精度在 5% 以内。通过优化和建模工作确定的工艺参数参数化控制,连续流分离产生了一种活性药物成分 (API),其单个杂质不超过 0.07%,分离产率为 74%。此外,新型 POD 系统首次满足 USP 盐酸环丙沙星专论的测定验收标准(在 98% 到 102% 之间)和水含量(在 4.7% 到 6.7% 之间)。
更新日期:2023-01-04
中文翻译:

通过连续两级结晶的系统工艺开发清除杂质
在新型便携式制造(按需制药)装置中部署了一种有条不紊的开发方法,以在美国药典 (USP) 专论和 ICH Q3A(R2) 新指南描述的含量、水含量和杂质规格范围内纯化盐酸环丙沙星水合物原料药中的杂质。一系列实验设计 (DOE) 和一次一个因素 (OFAT) 实验导致连续两阶段结晶的优化和控制,从而提高了盐酸环丙沙星水合物的纯度和收率。此外,在 20 °C 范围内批量导出具有统计学意义的线性模型,该模型跟踪纯化阶段 1 中难以清除的杂质的水平。该模型在连续流中进行了测试,预测的杂质去除精度在 5% 以内。通过优化和建模工作确定的工艺参数参数化控制,连续流分离产生了一种活性药物成分 (API),其单个杂质不超过 0.07%,分离产率为 74%。此外,新型 POD 系统首次满足 USP 盐酸环丙沙星专论的测定验收标准(在 98% 到 102% 之间)和水含量(在 4.7% 到 6.7% 之间)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号