当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Advances in Polyynes to Model Carbyne
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-12-09 , DOI: 10.1021/acs.accounts.2c00662 Yueze Gao 1 , Rik R Tykwinski 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-12-09 , DOI: 10.1021/acs.accounts.2c00662 Yueze Gao 1 , Rik R Tykwinski 1
Affiliation
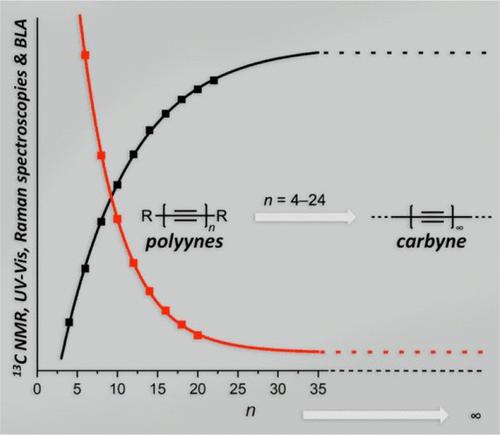
|
The formation and study of molecules that model the sp-hybridized carbon allotrope, carbyne, is a challenging field of synthetic physical organic chemistry. The target molecules, oligo- and polyynes, are often the preferred candidates as models for carbyne because they can be formed with monodisperse lengths as well as defined structures. Despite a simple linear structure, the synthesis of polyynes is often far from straightforward, due in large part to a highly conjugated framework that can render both precursors and products highly reactive, i.e., kinetically unstable. The vast majority of polyynes are formed as symmetrical products from terminal alkynes as precursors via an oxidative, acetylenic homocoupling reaction based on the Glaser, Eglinton–Galbraith, and Hay reactions. These reactions are very efficient for the synthesis of shorter polyynes (e.g., hexaynes and octaynes), but yields often drop dramatically as a function of length for longer derivatives, usually starting with the formation of decaynes. The most effective approach to circumvent unstable precursors and products has been through the incorporation of sterically demanding end groups that serve to “protect” the polyyne skeleton. This approach was arguably identified in the early 1950s by Bohlmann and co-workers with the synthesis of tBu-end-capped polyynes. During the next 50 years, a polyyne with 14 contiguous alkyne units remained the longest isolated derivative until 2010, when the record was extended to 22 alkyne units. The record length was broken again in 2020, when a polyyne consisting of 24 alkynes was isolated and characterized. Beyond polyynes, there have been several reports describing the potential synthesis of carbyne, but conclusive characterization and proof of structure have been tenuous. The sole example of synthetic carbyne arises from synthesis within carbon nanotubes, when chains of thousands of sp carbon atoms have been linked to form polydisperse samples of carbyne. Thus, model compounds for carbyne, the polyynes, remain the best means to examine and predict the experimental structure and properties of this carbon allotrope.
中文翻译:

Polyynes 的进展以模拟卡宾
模拟 sp2 杂化碳同素异形体卡宾的分子的形成和研究是合成物理有机化学的一个具有挑战性的领域。目标分子,寡炔和多炔,通常是卡宾模型的首选候选者,因为它们可以形成单分散长度和确定的结构。尽管具有简单的线性结构,但聚炔的合成通常远非直截了当,这在很大程度上是由于高度共轭的框架可以使前体和产物都具有高反应性,即动力学不稳定。绝大多数聚炔是由末端炔烃作为前体通过基于格拉泽、埃格林顿-加尔布雷思和海伊反应的氧化、炔属自偶联反应形成的对称产物。这些反应对于合成较短的多炔化合物(例如,己炔和辛炔)非常有效,但对于较长的衍生物,产率通常随着长度的变化而急剧下降,通常从衰烯的形成开始。规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合 规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合 规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合t Bu 封端的聚炔。在接下来的 50 年里,具有 14 个连续炔烃单元的聚炔一直是最长的孤立衍生物,直到 2010 年,该记录被扩展到 22 个炔烃单元。该记录长度在 2020 年再次被打破,当时分离并表征了由 24 个炔烃组成的聚炔。除了聚炔之外,还有几篇报道描述了卡宾的潜在合成,但结论性的表征和结构证明一直很脆弱。合成卡宾的唯一例子来自碳纳米管内的合成,当时数千个 sp 碳原子的链已连接形成卡宾的多分散样品。因此,卡宾的模型化合物,多炔,仍然是检查和预测这种碳同素异形体的实验结构和性质的最佳方法。
更新日期:2022-12-09
中文翻译:

Polyynes 的进展以模拟卡宾
模拟 sp2 杂化碳同素异形体卡宾的分子的形成和研究是合成物理有机化学的一个具有挑战性的领域。目标分子,寡炔和多炔,通常是卡宾模型的首选候选者,因为它们可以形成单分散长度和确定的结构。尽管具有简单的线性结构,但聚炔的合成通常远非直截了当,这在很大程度上是由于高度共轭的框架可以使前体和产物都具有高反应性,即动力学不稳定。绝大多数聚炔是由末端炔烃作为前体通过基于格拉泽、埃格林顿-加尔布雷思和海伊反应的氧化、炔属自偶联反应形成的对称产物。这些反应对于合成较短的多炔化合物(例如,己炔和辛炔)非常有效,但对于较长的衍生物,产率通常随着长度的变化而急剧下降,通常从衰烯的形成开始。规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合 规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合 规避不稳定前体和产物的最有效方法是通过加入用于“保护”多炔骨架的空间要求高的端基。这种方法可以说是在 1950 年代初期由 Bohlmann 及其同事通过综合t Bu 封端的聚炔。在接下来的 50 年里,具有 14 个连续炔烃单元的聚炔一直是最长的孤立衍生物,直到 2010 年,该记录被扩展到 22 个炔烃单元。该记录长度在 2020 年再次被打破,当时分离并表征了由 24 个炔烃组成的聚炔。除了聚炔之外,还有几篇报道描述了卡宾的潜在合成,但结论性的表征和结构证明一直很脆弱。合成卡宾的唯一例子来自碳纳米管内的合成,当时数千个 sp 碳原子的链已连接形成卡宾的多分散样品。因此,卡宾的模型化合物,多炔,仍然是检查和预测这种碳同素异形体的实验结构和性质的最佳方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号