当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed [3 + 2] Annulation of Aryl Halides with 7-Oxa- and 7-Azabenzonorbornadienes via C(sp2 or sp3)–H Activation
Organic Letters ( IF 5.2 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03422 Xiaojiao Li 1 , Xianting Pan 1 , Zisong Qi 1 , Xingwei Li 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03422 Xiaojiao Li 1 , Xianting Pan 1 , Zisong Qi 1 , Xingwei Li 1, 2
Affiliation
|
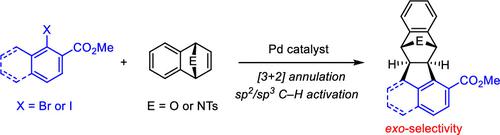
A series of epoxybenzo[k]fluoranthenes, epoxy-5H-benzo[b]fluorenes, and their aza analogues have been accessed via palladium-catalyzed exo-selective [3 + 2] annulation between aryl halides and 7-oxa- and 7-azabenzonorbornadienes. The reaction is initiated by the oxidative addition of a carbon–halogen bond, with intramolecular C(sp2 or sp3)–H activation being a key step. The enantioselective version of the reaction was also briefly explored.
中文翻译:

钯催化的 [3 + 2] 芳基卤化物与 7-Oxa- 和 7-氮杂苯并降冰片二烯通过 C(sp2 或 sp3)–H 活化环化
通过钯催化的芳基卤化物和 7-oxa- 和 7 之间的外选择性 [3 + 2] 环化,获得了一系列环氧苯并 [ k ] 荧蒽、环氧 -5 H -苯并 [ b ] 芴及其氮杂类似物-氮杂苯并降冰片二烯。该反应由碳-卤键的氧化加成引发,分子内 C(sp 2或 sp 3 )-H 活化是关键步骤。还简要探讨了该反应的对映选择性版本。
更新日期:2022-12-01
中文翻译:

钯催化的 [3 + 2] 芳基卤化物与 7-Oxa- 和 7-氮杂苯并降冰片二烯通过 C(sp2 或 sp3)–H 活化环化
通过钯催化的芳基卤化物和 7-oxa- 和 7 之间的外选择性 [3 + 2] 环化,获得了一系列环氧苯并 [ k ] 荧蒽、环氧 -5 H -苯并 [ b ] 芴及其氮杂类似物-氮杂苯并降冰片二烯。该反应由碳-卤键的氧化加成引发,分子内 C(sp 2或 sp 3 )-H 活化是关键步骤。还简要探讨了该反应的对映选择性版本。


























 京公网安备 11010802027423号
京公网安备 11010802027423号