当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
“Electron Complementation”-Induced Molybdenum Nitride/Co-Anchored Graphitic Carbon Nitride Porous Nanoparticles for Efficient Overall Water Splitting
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.inorgchem.2c03516 Beiyi Zhang 1 , Junqi Li 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.inorgchem.2c03516 Beiyi Zhang 1 , Junqi Li 1
Affiliation
|
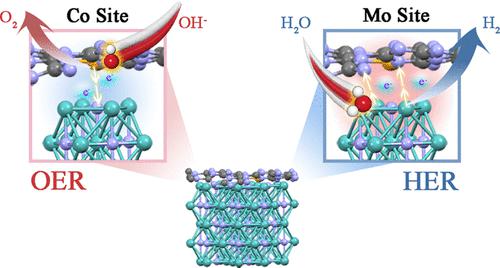
Maximizing the usable space of electrocatalysts and fine-tuning the interface geometry as well as the electronic structure to facilitate hydrogen and oxygen evolution reactions (HER and OER) have always been the focus of research. Herein, a homogeneous porous nanoparticle construction strategy was proposed, in which molybdenum nitride (Mo2N) particles were prepared by controlled heat treatment of the precursor nanoparticle induced by polyethylene glycol, and the Mo2N/Co-C3N4 heterostructure with a pore size of about 1.13 nm was obtained by compounding Co-anchored graphitic carbon nitride. In particular, exploring the change of charge distribution at the interface based on the principle of “electron complementation” shows that under the regulation of nitrogen with high electronegativity, the affinity of active site Co to oxygenated species in the OER process and the adsorption as well as cleavage ability of HER reactants in the active site were effectively optimized. Thus, Mo2N/Co-C3N4 not only inherits the functions of each component, but also provides an ideal heterogeneous interface for exhibiting impressive bifunctional activity, which only needs 100 and 210 mV to deliver 10 mA cm–2 for the HER and OER, respectively. In addition, the Mo2N/Co-C3N4 catalyst also demonstrates high overall water splitting stability with a slight current decrease after 95 h. Manipulating the electronic structure of multiple sites by constructing electronically complementary interfaces may provide another avenue to develop highly active catalysts for overall water splitting and other applications.
中文翻译:

“电子互补”诱导的氮化钼/共锚定石墨氮化碳多孔纳米颗粒用于有效的整体水分解
最大化电催化剂的可用空间和微调界面几何形状以及电子结构以促进析氢和析氧反应(HER 和 OER)一直是研究的重点。在此,提出了一种均质多孔纳米粒子构建策略,其中通过聚乙二醇诱导的前体纳米粒子的受控热处理制备氮化钼 (Mo 2 N) 粒子,Mo 2 N/Co-C 3 N 4通过复合Co锚定的石墨化碳氮化物获得了孔径约为1.13 nm的异质结构。特别是,基于“电子互补”原理探索界面电荷分布的变化表明,在高电负性氮的调节下,活性位点Co对OER过程中含氧物质的亲和力以及吸附有效优化了 HER 反应物在活性位点的裂解能力。因此,Mo 2 N/Co-C 3 N 4不仅继承了各组分的功能,而且还提供了理想的异质界面以展示令人印象深刻的双功能活性,仅需要 100 和 210 mV 即可提供 10 mA cm –2分别用于 HER 和 OER。此外,Mo 2 N/Co-C 3 N 4催化剂还表现出较高的整体水分解稳定性,95 小时后电流略有下降。通过构建电子互补界面来操纵多个位点的电子结构,可能为开发用于全水分解和其他应用的高活性催化剂提供另一种途径。
更新日期:2022-12-01
中文翻译:

“电子互补”诱导的氮化钼/共锚定石墨氮化碳多孔纳米颗粒用于有效的整体水分解
最大化电催化剂的可用空间和微调界面几何形状以及电子结构以促进析氢和析氧反应(HER 和 OER)一直是研究的重点。在此,提出了一种均质多孔纳米粒子构建策略,其中通过聚乙二醇诱导的前体纳米粒子的受控热处理制备氮化钼 (Mo 2 N) 粒子,Mo 2 N/Co-C 3 N 4通过复合Co锚定的石墨化碳氮化物获得了孔径约为1.13 nm的异质结构。特别是,基于“电子互补”原理探索界面电荷分布的变化表明,在高电负性氮的调节下,活性位点Co对OER过程中含氧物质的亲和力以及吸附有效优化了 HER 反应物在活性位点的裂解能力。因此,Mo 2 N/Co-C 3 N 4不仅继承了各组分的功能,而且还提供了理想的异质界面以展示令人印象深刻的双功能活性,仅需要 100 和 210 mV 即可提供 10 mA cm –2分别用于 HER 和 OER。此外,Mo 2 N/Co-C 3 N 4催化剂还表现出较高的整体水分解稳定性,95 小时后电流略有下降。通过构建电子互补界面来操纵多个位点的电子结构,可能为开发用于全水分解和其他应用的高活性催化剂提供另一种途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号