当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mild-Temperature Photothermal Effect Triggers Simultaneous Nitric Oxide- and Deferoxamine-Releasing Mesoporous Polydopamine-Based Nanoplatform for Robust Antibacterial, Anti-inflammation, and Wound-Healing Activity
Chemistry of Materials ( IF 8.6 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.chemmater.2c02686 Wenkang Liu 1 , Xiling Song 1 , Wei Liu 1 , Qi Lin 1 , Rui Xu 1 , Hui Li 1 , Wei Xue 1 , Siming Yu 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.chemmater.2c02686 Wenkang Liu 1 , Xiling Song 1 , Wei Liu 1 , Qi Lin 1 , Rui Xu 1 , Hui Li 1 , Wei Xue 1 , Siming Yu 1
Affiliation
|
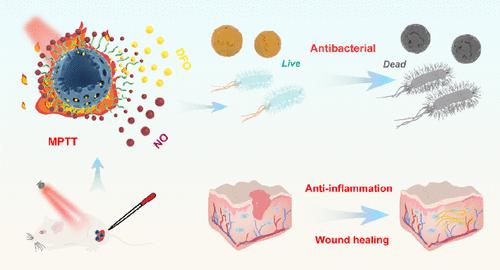
Currently, treatment of chronically infected wounds still remains a big challenge; thus, a novel strategy with a highly efficient therapeutic effect is of urgent demand. In the present work, we demonstrated that combinational use of nitric oxide (NO) and deferoxamine (DFO) is a promising way for treating hard-to-heal wounds. As a proof of concept, DFO was first loaded in mesoporous polydopamine (mPDA) and then functionalized by a chitosan-graft-third generation poly(amidoamine) polymer with terminal S-nitrosothiol groups (CP-SNO) via a strong electrostatic interaction, obtaining a multifunctional nanocomposite mPDA@DFO@CP-SNO. Upon near-infrared laser irradiation, mPDA@DFO@CP-SNO displayed a mild-temperature photothermal effect (MPTT) and a simultaneous NO and DFO controlled release property. The synergistic MPTT and NO antibacterial effect of mPDA@DFO@CP-SNO enabled effective elimination of both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus (S. aureus), as well as the biofilms formed by both bacteria. An in-depth mechanistic study revealed that mPDA@DFO@CP-SNO possessed particular binding affinity to the bacterial membrane, which significantly enhanced the damage effect on the bacterial membrane followed by boosting intracellular reactive oxygen species generation to accelerate GSH depletion and DNA dysfunction, finally leading to bacterial death. Moreover, the anti-inflammation and wound-healing effectiveness of mPDA@DFO@CP-SNO were demonstrated on an in vitro cell scratch model and an in vivoS. aureus-infected rat full-thickness skin wound model. Thanks to the triple therapeutic effects of MPTT, DFO, and NO, mPDA@DFO@CP-SNO significantly relieved the inflammation in rats’ infected wounds and promoted wound skin regeneration by upregulating expression of the hypoxia-inducible factor (HIF)-1α and the vascular endothelial growth factor.
中文翻译:

温和的光热效应触发同时释放一氧化氮和去铁胺的介孔聚多巴胺基纳米平台具有强大的抗菌、抗炎和伤口愈合活性
目前,慢性感染伤口的治疗仍然是一个很大的挑战;因此,迫切需要一种具有高效治疗效果的新策略。在目前的工作中,我们证明了联合使用一氧化氮 (NO) 和去铁胺 (DFO) 是治疗难以愈合伤口的一种很有前途的方法。作为概念验证,首先将 DFO 加载到介孔聚多巴胺 (mPDA) 中,然后通过具有末端 S-亚硝基硫醇基团 (CP-SNO) 的壳聚糖接枝第三代聚酰胺胺聚合物(CP-SNO)进行功能化强静电相互作用,获得多功能纳米复合材料mPDA@DFO@CP-SNO。在近红外激光照射下,mPDA@DFO@CP-SNO 表现出温和的光热效应 (MPTT) 以及同时控制 NO 和 DFO 的释放特性。mPDA@DFO@CP-SNO 的协同 MPTT 和 NO 抗菌作用能够有效消除革兰氏阴性大肠杆菌和革兰氏阳性金黄色葡萄球菌(S. aureus), 以及由两种细菌形成的生物膜。深入的机理研究表明,mPDA@DFO@CP-SNO 对细菌膜具有特殊的结合亲和力,显着增强了对细菌膜的损伤作用,随后促进细胞内活性氧的产生,加速 GSH 消耗和 DNA 功能障碍,最终导致细菌死亡。此外,mPDA@DFO@CP-SNO 的抗炎和伤口愈合效果在体外细胞划痕模型和体内金黄色葡萄球菌中得到证实-感染的大鼠全层皮肤伤口模型。得益于 MPTT、DFO 和 NO 的三重治疗作用,mPDA@DFO@CP-SNO 通过上调缺氧诱导因子 (HIF)-1α 和血管内皮生长因子。
更新日期:2022-12-01
中文翻译:

温和的光热效应触发同时释放一氧化氮和去铁胺的介孔聚多巴胺基纳米平台具有强大的抗菌、抗炎和伤口愈合活性
目前,慢性感染伤口的治疗仍然是一个很大的挑战;因此,迫切需要一种具有高效治疗效果的新策略。在目前的工作中,我们证明了联合使用一氧化氮 (NO) 和去铁胺 (DFO) 是治疗难以愈合伤口的一种很有前途的方法。作为概念验证,首先将 DFO 加载到介孔聚多巴胺 (mPDA) 中,然后通过具有末端 S-亚硝基硫醇基团 (CP-SNO) 的壳聚糖接枝第三代聚酰胺胺聚合物(CP-SNO)进行功能化强静电相互作用,获得多功能纳米复合材料mPDA@DFO@CP-SNO。在近红外激光照射下,mPDA@DFO@CP-SNO 表现出温和的光热效应 (MPTT) 以及同时控制 NO 和 DFO 的释放特性。mPDA@DFO@CP-SNO 的协同 MPTT 和 NO 抗菌作用能够有效消除革兰氏阴性大肠杆菌和革兰氏阳性金黄色葡萄球菌(S. aureus), 以及由两种细菌形成的生物膜。深入的机理研究表明,mPDA@DFO@CP-SNO 对细菌膜具有特殊的结合亲和力,显着增强了对细菌膜的损伤作用,随后促进细胞内活性氧的产生,加速 GSH 消耗和 DNA 功能障碍,最终导致细菌死亡。此外,mPDA@DFO@CP-SNO 的抗炎和伤口愈合效果在体外细胞划痕模型和体内金黄色葡萄球菌中得到证实-感染的大鼠全层皮肤伤口模型。得益于 MPTT、DFO 和 NO 的三重治疗作用,mPDA@DFO@CP-SNO 通过上调缺氧诱导因子 (HIF)-1α 和血管内皮生长因子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号