当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of Alkyne Hydroarylation Catalyzed by (P,C)-Cyclometalated Au(III) Complexes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10737 Marte Sofie Martinsen Holmsen 1, 2, 3 , Charlie Blons 1 , Abderrahmane Amgoune 1 , Matthieu Regnacq 4 , Denis Lesage 4 , E Daiann Sosa Carrizo 5 , Pierre Lavedan 6 , Yves Gimbert 4, 7 , Karinne Miqueu 5 , Didier Bourissou 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10737 Marte Sofie Martinsen Holmsen 1, 2, 3 , Charlie Blons 1 , Abderrahmane Amgoune 1 , Matthieu Regnacq 4 , Denis Lesage 4 , E Daiann Sosa Carrizo 5 , Pierre Lavedan 6 , Yves Gimbert 4, 7 , Karinne Miqueu 5 , Didier Bourissou 1
Affiliation
|
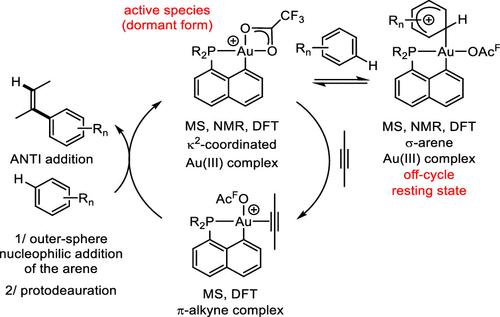
Over the last 5–10 years, gold(III) catalysis has developed rapidly. It often shows complementary if not unique features compared to gold(I) catalysis. While recent work has enabled major synthetic progress in terms of scope and efficiency, very little is yet known about the mechanism of Au(III)-catalyzed transformations and the relevant key intermediates have rarely been authenticated. Here, we report a detailed experimental/computational mechanistic study of the recently reported intermolecular hydroarylation of alkynes catalyzed by (P,C)-cyclometalated Au(III) complexes. The cationic (P,C)Au(OAcF)+ complex (OAcF = OCOCF3) was authenticated by mass spectrometry (MS) in the gas phase and multi-nuclear NMR spectroscopy in solution at low temperatures. According to density functional theory (DFT) calculations, the OAcF moiety is κ2-coordinated to gold in the ground state, but the corresponding κ1-forms featuring a vacant coordination site sit only slightly higher in energy. Side-on coordination of the alkyne to Au(III) then promotes nucleophilic addition of the arene. The energy profiles for the reaction between trimethoxybenzene (TMB) and diphenylacetylene (DPA) were computed by DFT. The activation barrier is significantly lower for the outer-sphere pathway than for the alternative inner-sphere mechanism involving C–H activation of the arene followed by migratory insertion. The π-complex of DPA was characterized by MS. An unprecedented σ-arene Au(III) complex with TMB was also authenticated both in the gas phase and in solution. The cationic complexes [(P,C)Au(OAcF)]+ and [(P,C)Au(OAcF)(σ-TMB)]+ stand as active species and off-cycle resting state during catalysis, respectively. This study provides a rational basis for the further development of Au(III) catalysis based on π-activation.
中文翻译:

(P,C)-环金属化 Au(III) 配合物催化炔氢芳基化反应的机理
在过去的 5-10 年里,金(III)催化发展迅速。与金 (I) 催化相比,它通常显示出互补(如果不是独特的)特征。虽然最近的工作在范围和效率方面取得了重大的合成进展,但人们对 Au(III) 催化转化的机制知之甚少,相关的关键中间体也很少得到验证。在这里,我们报告了最近报道的由 (P,C)-环金属化 Au(III) 配合物催化的炔烃分子间加氢芳基化的详细实验/计算机理研究。阳离子 (P,C)Au(OAc F ) +络合物 (OAc F = OCOCF 3) 通过气相质谱法 (MS) 和低温溶液中的多核 NMR 光谱法进行鉴定。根据密度泛函理论 (DFT) 计算,OAc F部分在基态与金配位为 κ 2 ,但相应的 κ 1-具有空置协调位点的形式的能量仅略高。然后炔烃与 Au(III) 的侧向配位促进芳烃的亲核加成。三甲氧基苯 (TMB) 和二苯乙炔 (DPA) 之间反应的能量分布由 DFT 计算。与涉及芳烃的 C-H 活化然后迁移插入的替代内球机制相比,外球途径的活化势垒明显较低。DPA 的 π 络合物通过 MS 表征。前所未有的 σ-芳烃 Au(III) 与 TMB 配合物也在气相和溶液中得到验证。阳离子络合物 [(P,C)Au(OAc F )] +和 [(P,C)Au(OAc F )(σ-TMB)] +在催化过程中分别作为活性物质和非循环静止状态。该研究为基于π-活化的Au(III)催化的进一步发展提供了合理的基础。
更新日期:2022-12-01
中文翻译:

(P,C)-环金属化 Au(III) 配合物催化炔氢芳基化反应的机理
在过去的 5-10 年里,金(III)催化发展迅速。与金 (I) 催化相比,它通常显示出互补(如果不是独特的)特征。虽然最近的工作在范围和效率方面取得了重大的合成进展,但人们对 Au(III) 催化转化的机制知之甚少,相关的关键中间体也很少得到验证。在这里,我们报告了最近报道的由 (P,C)-环金属化 Au(III) 配合物催化的炔烃分子间加氢芳基化的详细实验/计算机理研究。阳离子 (P,C)Au(OAc F ) +络合物 (OAc F = OCOCF 3) 通过气相质谱法 (MS) 和低温溶液中的多核 NMR 光谱法进行鉴定。根据密度泛函理论 (DFT) 计算,OAc F部分在基态与金配位为 κ 2 ,但相应的 κ 1-具有空置协调位点的形式的能量仅略高。然后炔烃与 Au(III) 的侧向配位促进芳烃的亲核加成。三甲氧基苯 (TMB) 和二苯乙炔 (DPA) 之间反应的能量分布由 DFT 计算。与涉及芳烃的 C-H 活化然后迁移插入的替代内球机制相比,外球途径的活化势垒明显较低。DPA 的 π 络合物通过 MS 表征。前所未有的 σ-芳烃 Au(III) 与 TMB 配合物也在气相和溶液中得到验证。阳离子络合物 [(P,C)Au(OAc F )] +和 [(P,C)Au(OAc F )(σ-TMB)] +在催化过程中分别作为活性物质和非循环静止状态。该研究为基于π-活化的Au(III)催化的进一步发展提供了合理的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号