Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2022-11-28 , DOI: 10.1016/j.cej.2022.140629 Tao Zeng , Shasha Xia , Shuqi Li , Xiuxiu Hong , Yashuang Wang , Lintuo Wang , Xinwen Huang
|
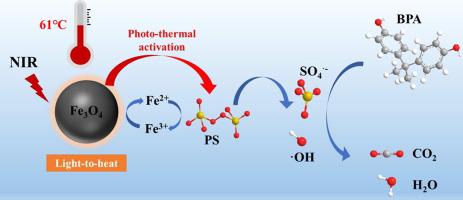
The utilization of renewable solar energy through near-infrared (NIR)-driven photothermal catalysts offers a potential novel strategy for environmental remediation. In this work, we reported an initial demonstration of the peroxydisulfate (PDS) activation in oxidative degradation of organics (represented by bisphenol A (BPA)) via a unique NIR light-to-heat conversion effect using cost-effective and biocompatible nanoparticles (NPs). The systematical investigation of the photothermal experiments found that Fe3O4 magnetic NPs exhibit great photothermal conversion efficiency (65.4%) and have the capability to quickly elevate the system temperature to 61℃. The increased reaction temperature leads to the homolytic fission of the O–O bond for producing sulfate radical (SO4•−) and the derivative hydroxyl radical (•OH), as confirmed by the radical quenching and electron paramagnetic resonances (EPR) tests, and in turn accelerates the thermocatalytic degradation reaction of BPA. The BPA degradation reaction rate of the NIR/Fe3O4/PDS system is about 25.8 times and 9.4 times of that in the NIR/PDS and Fe3O4/PDS systems, respectively. Both photothermal heating and surface valence states conversion of Fe2+/Fe3+ on Fe3O4 under dark conditions make a contribution to the observed oxidation reactions. Moreover, this NIR light-to-heat activation system exhibits efficient photocatalytic degradation of BPA molecules in deep water by virtue of the deep penetration capability of NIR light in water, highlighting NIR-driven photothermal catalysts hold the excellent potential to be applied in practical water restoration through PDS activation.
中文翻译:

近红外光驱动过二硫酸盐与 Fe3O4 热催化活化氧化有机物的动力学和机理研究
通过近红外 (NIR) 驱动的光热催化剂利用可再生太阳能为环境修复提供了一种潜在的新策略。在这项工作中,我们报道了通过使用具有成本效益和生物相容性的纳米粒子 (NPs)的独特 NIR 光热转换效应,过二硫酸盐 (PDS) 在有机物(以双酚 A (BPA) 为代表)氧化降解过程中的活化作用的初步证明). 光热实验的系统研究发现,Fe 3 O 4磁性 NPs 表现出很高的光热转换效率 (65.4%),并具有将系统温度快速升高至 61℃ 的能力。升高的反应温度导致 O-O 键的均裂裂变产生硫酸根 (SO 4 •- ) 和衍生的羟基自由基 (•OH),正如自由基淬灭和电子顺磁共振 (EPR) 测试所证实的那样,进而加速BPA的热催化降解反应。NIR/Fe 3 O 4 /PDS体系的BPA降解反应速率分别是NIR/PDS和Fe 3 O 4 / PDS体系的25.8倍和9.4倍。Fe的光热加热和表面价态转换黑暗条件下Fe 3 O 4上的2+ /Fe 3+对观察到的氧化反应有贡献。此外,该NIR光热活化系统凭借NIR光在水中的深度穿透能力,在深水中表现出高效的BPA分子光催化降解,突出了NIR驱动的光热催化剂在实际水中的应用潜力通过 PDS 激活恢复。


























 京公网安备 11010802027423号
京公网安备 11010802027423号