当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dearomatization of Biaryls through Polarity Mismatched Radical Spirocyclization
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-12-01 , DOI: 10.1002/anie.202215422 Carlos R Azpilcueta-Nicolas 1 , Derek Meng 1 , Simon Edelmann 1 , Jean-Philip Lumb 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-12-01 , DOI: 10.1002/anie.202215422 Carlos R Azpilcueta-Nicolas 1 , Derek Meng 1 , Simon Edelmann 1 , Jean-Philip Lumb 1
Affiliation
|
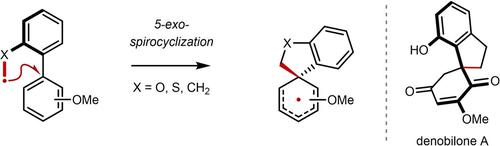
A redox-neutral dearomatization transforms biaryls into complex spirocycles commonly found in bioactive natural products. This polarity mismatched radical spirocyclization tolerates diverse functional groups and is applied in the regioselective synthesis of 2,4- and 2,5-cyclohexadienones. The first synthesis of denobilone A, a cytotoxic plant metabolite, showcases its utility to access underexplored three-dimensional chemical space.
中文翻译:

通过极性不匹配的自由基螺环化联芳烃脱芳构化
氧化还原中性脱芳构化将联芳基转化为生物活性天然产物中常见的复杂螺环。这种极性不匹配的自由基螺环化可容忍不同的官能团,并应用于 2,4- 和 2,5- 环己二烯酮的区域选择性合成。地诺比龙 A(一种细胞毒性植物代谢物)的首次合成展示了其在进入未充分探索的三维化学空间方面的实用性。
更新日期:2022-12-01
中文翻译:

通过极性不匹配的自由基螺环化联芳烃脱芳构化
氧化还原中性脱芳构化将联芳基转化为生物活性天然产物中常见的复杂螺环。这种极性不匹配的自由基螺环化可容忍不同的官能团,并应用于 2,4- 和 2,5- 环己二烯酮的区域选择性合成。地诺比龙 A(一种细胞毒性植物代谢物)的首次合成展示了其在进入未充分探索的三维化学空间方面的实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号