当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Photocatalytic Splitting of Carbon Dioxide Using Co-cationic Perovskite Nanocrystals in the Absence of Water
ACS Energy Letters ( IF 22.0 ) Pub Date : 2022-11-30 , DOI: 10.1021/acsenergylett.2c02342 Sumit S. Bhosale, Aparna K. Kharade, Sudhakar Narra, Sue-min Chang, Eric Wei-Guang Diau
ACS Energy Letters ( IF 22.0 ) Pub Date : 2022-11-30 , DOI: 10.1021/acsenergylett.2c02342 Sumit S. Bhosale, Aparna K. Kharade, Sudhakar Narra, Sue-min Chang, Eric Wei-Guang Diau
|
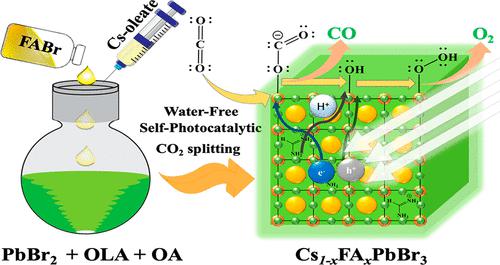
Environmental concerns demand efficient removal of CO2, a major greenhouse gas. For this purpose, a traditional chemical strategy implements a catalytic conversion of CO2 to CO, with H2O as the sacrificial agent to generate O2. Herein we report the first self-photocatalytic conversion of CO2 to generate CO and O2 in the absence of H2O, using co-cationic perovskite nanocrystal Cs0.55FA0.45PbBr3. We obtained a record production rate 105 μmol g–1 h–1 of CO, which is three times that with CsPbBr3 as photocatalyst at the gas–solid interface. During photocatalytic reaction, a phase transition occurred with an enlarged crystal size through the effect of Ostwald ripening, for which the CO yield approached 3.1 mmol g–1 within the reaction period of ∼60 h. During this process, both FA and oleylammonium cations were released to provide the proton source for the CO2 reduction to proceed and generate hydroxyl species required for oxidation. A self-photocatalysis mechanism involving bound hydroxyls is proposed.
中文翻译:

在无水条件下使用共阳离子钙钛矿纳米晶体自光催化分解二氧化碳
环境问题要求有效去除主要温室气体 CO 2。为此,传统的化学策略将 CO 2催化转化为CO,并以 H 2 O 作为牺牲剂生成 O 2。在此,我们报告了在没有 H 2 O 的情况下使用共阳离子钙钛矿纳米晶体 Cs 0.55 FA 0.45 PbBr 3将 CO 2首次自光催化转化为CO 和 O 2。我们获得了 105 μmol g –1 h –1 CO的创纪录生产率,是 CsPbBr 3的三倍作为气固界面的光催化剂。在光催化反应过程中,通过奥斯特瓦尔德熟化的作用发生相变,晶体尺寸增大,在~60 小时的反应时间内, CO 产率接近 3.1 mmol g -1 。在此过程中,FA 和油基铵阳离子均被释放,为 CO 2还原提供质子源以进行并产生氧化所需的羟基物质。提出了一种涉及结合羟基的自光催化机制。
更新日期:2022-11-30
中文翻译:

在无水条件下使用共阳离子钙钛矿纳米晶体自光催化分解二氧化碳
环境问题要求有效去除主要温室气体 CO 2。为此,传统的化学策略将 CO 2催化转化为CO,并以 H 2 O 作为牺牲剂生成 O 2。在此,我们报告了在没有 H 2 O 的情况下使用共阳离子钙钛矿纳米晶体 Cs 0.55 FA 0.45 PbBr 3将 CO 2首次自光催化转化为CO 和 O 2。我们获得了 105 μmol g –1 h –1 CO的创纪录生产率,是 CsPbBr 3的三倍作为气固界面的光催化剂。在光催化反应过程中,通过奥斯特瓦尔德熟化的作用发生相变,晶体尺寸增大,在~60 小时的反应时间内, CO 产率接近 3.1 mmol g -1 。在此过程中,FA 和油基铵阳离子均被释放,为 CO 2还原提供质子源以进行并产生氧化所需的羟基物质。提出了一种涉及结合羟基的自光催化机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号