当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
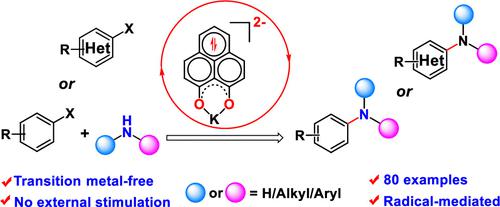
Reduced-Phenalenyl-Based Molecule as a Super Electron Donor for Radical-Mediated C–N Coupling Catalysis at Room Temperature
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09225 Swagata Sil 1 , Athul Santha Bhaskaran 2 , Soumi Chakraborty 1 , Bhagat Singh 1 , Rositha Kuniyil 2 , Swadhin K Mandal 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09225 Swagata Sil 1 , Athul Santha Bhaskaran 2 , Soumi Chakraborty 1 , Bhagat Singh 1 , Rositha Kuniyil 2 , Swadhin K Mandal 1
Affiliation
|
We demonstrate that an in situ generated di-reduced phenalenyl (PLY) species accumulates sufficiently high energy and acts as a super electron donor to generate aryl radicals from aryl halides to accomplish Buchwald–Hartwig-type C–N cross-coupling reactions at room temperature. This catalytic protocol does not require any external stimuli such as heat, light, or cathodic current. This protocol shows a wide variety of substrate scope covering different genres of aryl and heteroaryl halides with various aromatic as well as aliphatic amines and late-stage functionalization of the well-known natural products. The control experiments, along with extensive density functional theory (DFT) calculations, unveil that the aryl radical is generated by a single electron transfer from the di-reduced PLY to the aryl halide substrate. The aryl radical acts as an electrophile and binds with amine, leading to the chemically driven radical-mediated C–N cross-coupling under transition-metal-free conditions.
中文翻译:

还原苯二酚基分子作为室温下自由基介导的 C-N 偶联催化的超级电子供体
我们证明原位生成的双还原苯二甲酰 (PLY) 物种积累了足够高的能量,并作为超级电子供体从芳基卤化物中产生芳基自由基,从而在室温下完成 Buchwald-Hartwig 型 C-N 交叉偶联反应. 这种催化方案不需要任何外部刺激,如热、光或阴极电流。该协议显示了各种各样的底物范围,涵盖不同类型的芳基和杂芳基卤化物以及各种芳香胺和脂肪胺以及著名天然产物的后期功能化。控制实验以及广泛的密度泛函理论 (DFT) 计算揭示了芳基自由基是由从双还原 PLY 到芳基卤化物底物的单个电子转移产生的。
更新日期:2022-11-30
中文翻译:

还原苯二酚基分子作为室温下自由基介导的 C-N 偶联催化的超级电子供体
我们证明原位生成的双还原苯二甲酰 (PLY) 物种积累了足够高的能量,并作为超级电子供体从芳基卤化物中产生芳基自由基,从而在室温下完成 Buchwald-Hartwig 型 C-N 交叉偶联反应. 这种催化方案不需要任何外部刺激,如热、光或阴极电流。该协议显示了各种各样的底物范围,涵盖不同类型的芳基和杂芳基卤化物以及各种芳香胺和脂肪胺以及著名天然产物的后期功能化。控制实验以及广泛的密度泛函理论 (DFT) 计算揭示了芳基自由基是由从双还原 PLY 到芳基卤化物底物的单个电子转移产生的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号