当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NIS-Promoted Selective Amino-Diazidation and Amino-Iodoazidation of O-Homoallyl Benzimidates: Synthesis of Vicinal Diazido 1,3-Oxazines and Vicinal Iodoazido 1,3-Oxazines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.joc.2c02252 Tong-Yang Cao 1 , Lin Qi 1 , Wei Dong 1 , Zhi-Min Yan 1 , Shi-Chao Ji 1 , Jian-Long Du 1 , Linlin Zhang 1 , Wei Li 1 , Li-Jing Wang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.joc.2c02252 Tong-Yang Cao 1 , Lin Qi 1 , Wei Dong 1 , Zhi-Min Yan 1 , Shi-Chao Ji 1 , Jian-Long Du 1 , Linlin Zhang 1 , Wei Li 1 , Li-Jing Wang 1
Affiliation
|
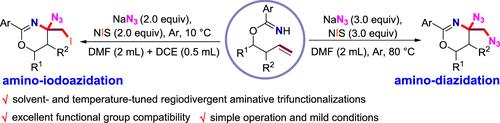
Amines, especially those with multi-nitrogen moieties, are widespread in natural products and biologically active compounds. Thus, the development of direct and efficient methods to introduce multiple nitrogen-containing fragments into compounds in one step is highly desirable yet challenging. Herein, we report an NIS-promoted selective amino-diazidation and amino-iodoazidation of O-homoallyl benzimidates with NaN3. By using this protocol, a variety of vicinal diazido-substituted 1,3-oxazines and vicinal iodoazido-substituted 1,3-oxazines were directly synthesized in a controllable manner. Preliminary mechanistic investigations revealed that the reaction operates through a NIS-promoted four-step cascade process. The developed method has the merits of metal-free, excellent functional group compatibility, simple operation, and mild conditions.
中文翻译:

NIS 促进的 O-Homoallyl Benzimidates 的选择性氨基二叠氮化和氨基碘叠氮化:邻位二叠氮基 1,3-恶嗪和邻位碘叠氮基 1,3-恶嗪的合成
胺类,尤其是具有多氮部分的胺类,广泛存在于天然产物和生物活性化合物中。因此,非常需要开发一种直接有效的方法,将多个含氮片段一步引入化合物中,但也具有挑战性。在此,我们报告了 NIS 促进的O -homoallyl benzimidates 与 NaN 3的选择性氨基二叠氮化和氨基碘叠氮化. 通过使用该协议,以可控方式直接合成了多种邻位二叠氮基取代的 1,3-恶嗪和邻位碘叠氮基取代的 1,3-恶嗪。初步的机理研究表明,该反应通过 NIS 促进的四步级联过程进行。该方法具有无金属、官能团相容性好、操作简单、条件温和等优点。
更新日期:2022-11-30
中文翻译:

NIS 促进的 O-Homoallyl Benzimidates 的选择性氨基二叠氮化和氨基碘叠氮化:邻位二叠氮基 1,3-恶嗪和邻位碘叠氮基 1,3-恶嗪的合成
胺类,尤其是具有多氮部分的胺类,广泛存在于天然产物和生物活性化合物中。因此,非常需要开发一种直接有效的方法,将多个含氮片段一步引入化合物中,但也具有挑战性。在此,我们报告了 NIS 促进的O -homoallyl benzimidates 与 NaN 3的选择性氨基二叠氮化和氨基碘叠氮化. 通过使用该协议,以可控方式直接合成了多种邻位二叠氮基取代的 1,3-恶嗪和邻位碘叠氮基取代的 1,3-恶嗪。初步的机理研究表明,该反应通过 NIS 促进的四步级联过程进行。该方法具有无金属、官能团相容性好、操作简单、条件温和等优点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号