当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evidence for Oxonium Ions in Ethereal “Hydrogen Chloride”
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03622 Corin C Wagen 1 , Eric N Jacobsen 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03622 Corin C Wagen 1 , Eric N Jacobsen 1
Affiliation
|
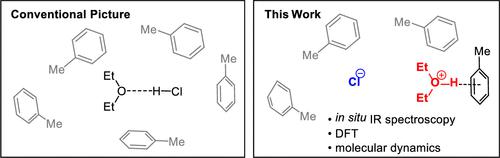
Although solutions of hydrogen chloride in ethereal solvents like diethyl ether or dioxane are commonly employed in the laboratory, the solution structure of these reagents has yet to be firmly established. Here, we analyze solutions of ethereal hydrogen chloride or deuterium chloride in toluene, in dichloromethane, or in the absence of a co-solvent by in situ infrared spectroscopy. The resulting spectra are inconsistent with free HCl or often-proposed 1:1 HCl–ether complexes but closely match the predicted spectra of oxonium ions generated via protonation of diethyl ether. Molecular dynamics simulation of the oxonium chloride complexes provides evidence for an outer-sphere contact ion pair. These results suggest new approaches for tuning the acidity of strong Brønsted acids in organic solvents and demonstrate the importance of conducting spectroscopic measurements under reaction-relevant conditions.
中文翻译:

以太“氯化氢”中氧鎓离子的证据
虽然氯化氢在乙醚或二恶烷等醚类溶剂中的溶液在实验室中很普遍,但这些试剂的溶液结构尚未确定。在这里,我们分析了乙醚氯化氢或氯化氘在甲苯、二氯甲烷或没有助溶剂的情况下的原位溶液红外光谱。所得光谱与游离 HCl 或通常提出的 1:1 HCl-醚络合物不一致,但与乙醚质子化产生的氧鎓离子的预测光谱非常匹配。氧鎓氯化物配合物的分子动力学模拟为外层接触离子对提供了证据。这些结果提出了调整有机溶剂中强布朗斯台德酸酸度的新方法,并证明了在反应相关条件下进行光谱测量的重要性。
更新日期:2022-11-30
中文翻译:

以太“氯化氢”中氧鎓离子的证据
虽然氯化氢在乙醚或二恶烷等醚类溶剂中的溶液在实验室中很普遍,但这些试剂的溶液结构尚未确定。在这里,我们分析了乙醚氯化氢或氯化氘在甲苯、二氯甲烷或没有助溶剂的情况下的原位溶液红外光谱。所得光谱与游离 HCl 或通常提出的 1:1 HCl-醚络合物不一致,但与乙醚质子化产生的氧鎓离子的预测光谱非常匹配。氧鎓氯化物配合物的分子动力学模拟为外层接触离子对提供了证据。这些结果提出了调整有机溶剂中强布朗斯台德酸酸度的新方法,并证明了在反应相关条件下进行光谱测量的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号