当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 4-Imidazolidinones from Diamides and Ethynyl Benziodoxolones via Double Michael-Type Addition: Ethynyl Benziodoxolones as Electrophilic Ynol Synthons
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.orglett.2c03648 Ayaka Shimizu 1 , Atsushi Shibata 1 , Takashi Kano 1 , Yuuichi Kumai 1 , Ryouhei Kawakami 1 , Hiroyoshi Esaki 2 , Kazuaki Fukushima 2 , Norihiro Tada 1 , Akichika Itoh 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.orglett.2c03648 Ayaka Shimizu 1 , Atsushi Shibata 1 , Takashi Kano 1 , Yuuichi Kumai 1 , Ryouhei Kawakami 1 , Hiroyoshi Esaki 2 , Kazuaki Fukushima 2 , Norihiro Tada 1 , Akichika Itoh 1
Affiliation
|
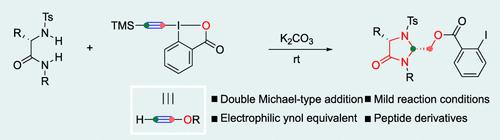
The moiety of 4-imidazolidinone is an important structural motif in organic synthesis and medicinal chemistry. We present the synthesis of 4-imidazolidinones from various diamides with ethynyl benziodoxolones through double Michael-type addition, which is an unprecedented reaction mode for hypervalent alkynyl iodine compounds. cis-2,5-Disubstituted 4-imidazolidinones were diastereoselectively synthesized from amino acid derived diamides. Having derivatized the 4-imidazolidinones, several control experiments and density functional theory calculations were conducted to realize mechanistic insight.
中文翻译:

通过双迈克尔型加成从二酰胺和乙炔基苯并氧唑酮合成 4-咪唑啉酮:作为亲电子 Ynol 合成子的乙炔基苯并氧唑酮
4-咪唑啉酮部分是有机合成和药物化学中的重要结构基序。我们展示了通过双迈克尔型加成从各种二酰胺与乙炔基苯并氧酮合成 4-咪唑啉酮,这是高价炔基碘化合物前所未有的反应模式。顺式-2,5-二取代的 4-咪唑啉酮是从氨基酸衍生的二酰胺中非对映选择性合成的。将 4-咪唑啉酮衍生化后,进行了几次控制实验和密度泛函理论计算以实现机理洞察力。
更新日期:2022-11-28
中文翻译:

通过双迈克尔型加成从二酰胺和乙炔基苯并氧唑酮合成 4-咪唑啉酮:作为亲电子 Ynol 合成子的乙炔基苯并氧唑酮
4-咪唑啉酮部分是有机合成和药物化学中的重要结构基序。我们展示了通过双迈克尔型加成从各种二酰胺与乙炔基苯并氧酮合成 4-咪唑啉酮,这是高价炔基碘化合物前所未有的反应模式。顺式-2,5-二取代的 4-咪唑啉酮是从氨基酸衍生的二酰胺中非对映选择性合成的。将 4-咪唑啉酮衍生化后,进行了几次控制实验和密度泛函理论计算以实现机理洞察力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号