当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
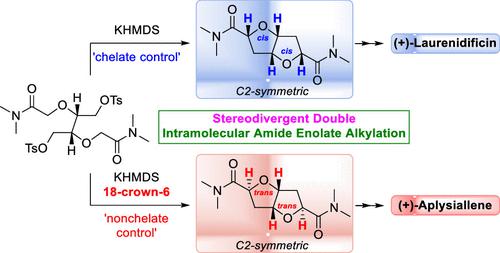
Highly Stereodivergent Construction of a C2-Symmetric cis,cis- and trans,trans-2,6-Dioxabicyclo[3.3.0]octane Framework by Double Intramolecular Amide Enolate Alkylation: Total Synthesis of (+)-Laurenidificin and (+)-Aplysiallene
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03494 Iljin Shin 1 , Hongjun Jang 1 , Soo Yeon Kwak 1 , Youngjik Park 1 , Dongjoo Lee 1 , Hyoungsu Kim 1 , Deukjoon Kim 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03494 Iljin Shin 1 , Hongjun Jang 1 , Soo Yeon Kwak 1 , Youngjik Park 1 , Dongjoo Lee 1 , Hyoungsu Kim 1 , Deukjoon Kim 2
Affiliation
|
The highly stereoselective construction of C2-symmetric cis,cis- and trans,trans-2,6-dioxabicyclo[3.3.0]octane (fused bis-tetrahydrofuran) skeletons 4a and 4b has been accomplished via a novel stereodivergent double intramolecular amide enolate alkylation of common cyclization substrate 5 through the judicious choice of “chelate” versus crown ether-promoted “nonchelate” control. Application of this methodology has provided access to substrate-controlled concise total syntheses of (+)-laurenidificin (3) and (+)-aplysiallene (ent-2), which possess cis/cis- and trans/trans-fused bis-tetrahydrofuran cores, respectively.
中文翻译:

双分子内酰胺烯醇烷基化 C2-对称顺式、顺式和反式、反式 2,6-二氧杂双环 [3.3.0] 辛烷骨架的高度立体发散结构:(+)-Laurenidificin 和 (+)-Aplysiallene 的全合成
C 2 -对称顺式、顺式-和反式、反式-2,6-二氧杂双环[3.3.0]辛烷(稠合双-四氢呋喃)骨架4a和4b的高度立体选择性构建已通过新型立体发散双分子内酰胺烯醇化物完成通过明智地选择“螯合”与冠醚促进的“非螯合”控制对常见环化底物5进行烷基化。该方法的应用提供了 (+)-laurenidificin ( 3 ) 和 (+)-aplysiallene ( ent - 2 ) 的底物控制简明全合成途径), 分别具有顺式/顺式和反式/反式稠合双四氢呋喃核。
更新日期:2022-11-30
中文翻译:

双分子内酰胺烯醇烷基化 C2-对称顺式、顺式和反式、反式 2,6-二氧杂双环 [3.3.0] 辛烷骨架的高度立体发散结构:(+)-Laurenidificin 和 (+)-Aplysiallene 的全合成
C 2 -对称顺式、顺式-和反式、反式-2,6-二氧杂双环[3.3.0]辛烷(稠合双-四氢呋喃)骨架4a和4b的高度立体选择性构建已通过新型立体发散双分子内酰胺烯醇化物完成通过明智地选择“螯合”与冠醚促进的“非螯合”控制对常见环化底物5进行烷基化。该方法的应用提供了 (+)-laurenidificin ( 3 ) 和 (+)-aplysiallene ( ent - 2 ) 的底物控制简明全合成途径), 分别具有顺式/顺式和反式/反式稠合双四氢呋喃核。


























 京公网安备 11010802027423号
京公网安备 11010802027423号