当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Highly Enantio- and Diastereoselective Alkenylation of β,γ-Unsaturated Butenolides via Dynamic Kinetic Resolution
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03551 Xiaosa Lu 1 , Jie Zhu 1 , Yinhua Huang 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03551 Xiaosa Lu 1 , Jie Zhu 1 , Yinhua Huang 2
Affiliation
|
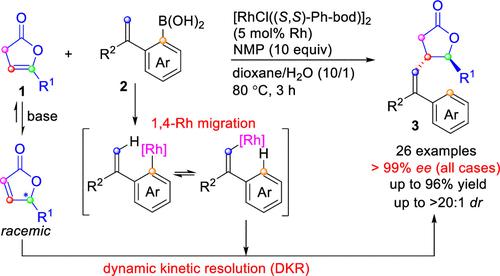
A rhodium-catalyzed highly enantio- and diastereoselective alkenylation of β,γ-unsaturated butenolides is reported. The use of a chiral diene ligand, (S,S)-Ph-bod, enables the facile synthesis of chiral butyrolactones in high yields with extremely high enantioselectivities (>99% ee in all cases) and high diastereoselectivities (up to >20:1 dr). The key process of the reaction involves the isomerization of β,γ-unsaturated butenolides to racemic α,β-unsaturated butenolides and the subsequent dynamic kinetic resolution through ligand-controlled, enantioselective alkenylation with an alkenylrhodium species that is generated in situ via 1,4-rhodium migration.
中文翻译:

铑催化的 β,γ-不饱和丁烯酸内酯的高度对映和非对映选择性烯基化通过动态动力学拆分
报道了一种铑催化的 β,γ-不饱和丁烯酸内酯的高度对映和非对映选择性烯基化反应。使用手性二烯配体 ( S , S )-Ph-bod,能够以高产率轻松合成手性丁内酯,具有极高的对映选择性(在所有情况下 >99% ee)和高非对映选择性(高达 >20: 1 博士)。反应的关键过程包括 β,γ-不饱和丁烯酸内酯异构化为外消旋 α,β-不饱和丁烯酸内酯,以及随后通过配体控制的动态动力学拆分,通过 1,4 原位生成的烯基铑物质进行对映选择性烯基化- 铑迁移。
更新日期:2022-11-30
中文翻译:

铑催化的 β,γ-不饱和丁烯酸内酯的高度对映和非对映选择性烯基化通过动态动力学拆分
报道了一种铑催化的 β,γ-不饱和丁烯酸内酯的高度对映和非对映选择性烯基化反应。使用手性二烯配体 ( S , S )-Ph-bod,能够以高产率轻松合成手性丁内酯,具有极高的对映选择性(在所有情况下 >99% ee)和高非对映选择性(高达 >20: 1 博士)。反应的关键过程包括 β,γ-不饱和丁烯酸内酯异构化为外消旋 α,β-不饱和丁烯酸内酯,以及随后通过配体控制的动态动力学拆分,通过 1,4 原位生成的烯基铑物质进行对映选择性烯基化- 铑迁移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号